Population Estimates for Age-Related Physiology
The implementation of PBPK in GastroPlus® includes an internal module named PEAR Physiology™ (Population Estimates for Age-Related Physiology). This module calculates organ physiologies for American (Western), Japanese (Asian), and Chinese human models across ages ranging from premature neonates to 85 years old. Additionally, it provides adult-only organ physiologies for several commonly used pre-clinical species.
The age-related population data in the PEAR™ module includes data for body weight, height, body mass index, and bioelectrical impedance resistance measured at 50 KHz. The data for the PEAR™ module is sourced as follows:
For humans:
The age-related population data for 11,039 Americans (50% male and 50% female) comes from the National Health and Nutrition Examination Survey (NHANES): www.cdc.gov/nchs/nhanes/index.html).
The age-related population data for Japanese comes from a paper by Ogiu et al. 0
The age-related population data for Chinese was collected from over 100 publications, with the primary sources referenced here 0 0 0 0 0 0 0 0 0 0 .
For pre-clinical species:
The default rat physiology comes from a paper on PBPK modeling of terbinafine 0 .
The default physiologies for dog, mouse, monkey, and rabbit are based mainly on the data published by Brown 0 and Davies 0 .
Additional information was supplied from number of other publications for the following species:
Dog 0 0 0
Mouse 0 0 0
Monkey 0 0 0
Rabbit 0 0 0 0 0 0 0 0 0
Individual organ physiology models are beyond the scope of this discussion; however, much of the thought process for the steps for generating organ physiology was influenced by Price et al. 0 and Haddad et al. 0
Look up average weight, height, and bioimpedance.
Calculate BMI and Fat-Free Mass (FFM).
Set the constant perfusion rates per mL tissue. (Table 13 in the referenced Price manuscript.)
Calculate blood volumes 0 .
Calculate weight, volume, density, and perfusion for each tissue.
Infant physiologies
The infant physiology algorithms in GastroPlus® cover infants less than 1 year old, including newborns born up to 16 weeks premature. The core information for individual parameters (body height and weight, tissue sizes, and so on) was obtained from ICRP publication 23 0 and was supplemented by data from additional publications.
For the total body height and weight, as well as the majority of tissue sizes, there appears to be a continuous progression of growth from fetus to neonate and infant. Birth does not appear to be a significant event and the development rate is dependent only on post-menstrual age (PMA), where PMA = gestational age + postnatal age. For these cases, smooth relationships between the size and PMA covering the entire range of ages from a 16 week premature newborn to a term-born 1-year-old infant were found and implemented in GastroPlus®. See Figure 2-17 through Figure 2-26.
Some of the physiological parameters, however, are dependent separately on gestational age and postnatal age. For example, premature neonates and infants experience a “catch-up” period during the first few weeks and the percent of fat mass in their bodies increases faster (Figure 2-27) than in their term-born counterparts (neonates and infants of the same post-menstrual age born at term). Similarly, the hematocrit (Figure 2-38) and glomerular filtration rate (Figure 2-49) ontogeny change at birth, and as a result, gestational age and postnatal age must be considered separately when estimating these values.
The majority of the observed physiological parameters used to derive the algorithms for infant physiologies were for infants from western populations. Currently, the same equations are also being used for infants from Japanese and Chinese populations.
Although individual organ physiology models are beyond the scope of this discussion, example plots of experimental data and relationships implemented in GastroPlus® for some of the tissue sizes, compositions, and some of the physiological parameters are shown in the following sections. See:
Infant body and tissue sizes
For infants with PMA < 39 weeks, the weight and height data were not available from survey data. Instead, they were extracted from multiple publications, which included both Chinese and Caucasian infants. The same bivariate distribution function for weight and height is used for both males and females across Chinese, Caucasian, and Japanese infants in this age range.
Body weight and height
Figure 2-17: Body weight and height for preterm/term infants up to 42 weeks old
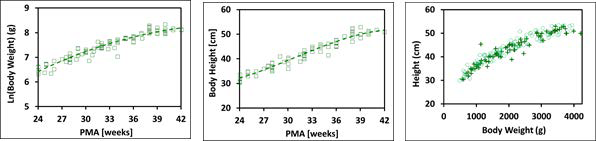
The plot on the left and the plot in the middle show log normal body weight and body height, respectively versus post-menstrual age (PMA). The data points represent experimental data 0 0 0 0 0 0 0 0 0 0 0 . The plotted lines show tissue weights and heights calculated using equations implemented in GastroPlus®. The plot on the right compares the observed distribution of body weights and heights (cross) in these infants with the distribution of body weights and heights generated by bivariate distribution function implemented in GastroPlus® (circles).
Figure 2-18: Body weight and height for term infants up to 1 year old
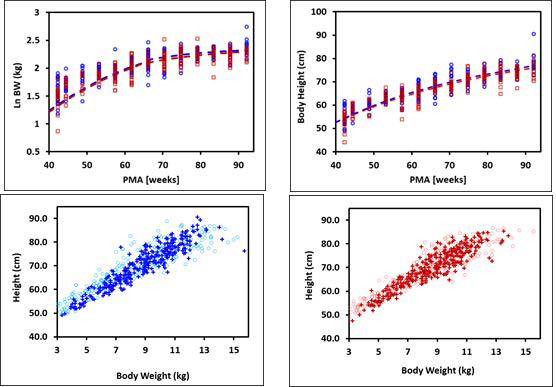
The plots on the top show log normal body weight (left) and body height (right) versus post- menstrual age (PMA). The data points represent NHANES 2003-2004 survey data and the lines show body weights and heights calculated using equations implemented in GastroPlus®. The data for males and females are shown in blue and red, respectively. The plots on the bottom compare the observed distributions of body weights and heights (cross) in these infants with the distribution of body weights and heights generated by bivariate distribution function implemented in GastroPlus® (circles) for males (left) and females (right).
Tissue weight versus post-menstrual age (PMA) for infants up to one year old
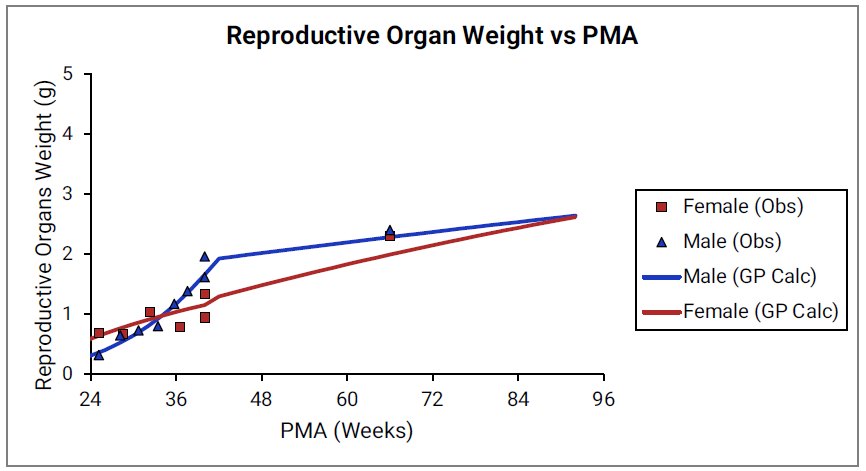
For Figure 2-19 through Figure 2-26, the data points represent experimental data 0 . The plotted lines represent tissue weights calculated using equations in GastroPlus®. The data for males and females are shown in blue and red, respectively. Tissue weights for all tissues other than muscle and reproductive organs that were calculated using previously published equations for healthy Danish infants 0 are shown (gray circles) for comparison.
Figure 2-19: Liver weight versus PMA
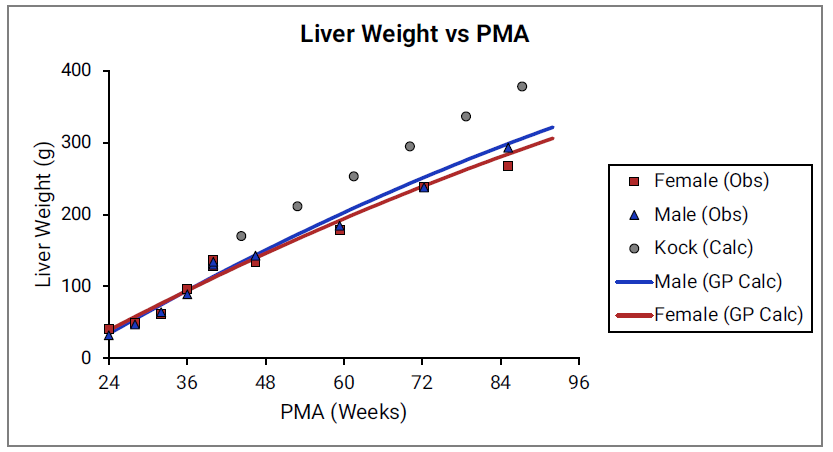
Figure 2-20: Kidney weight versus PMA
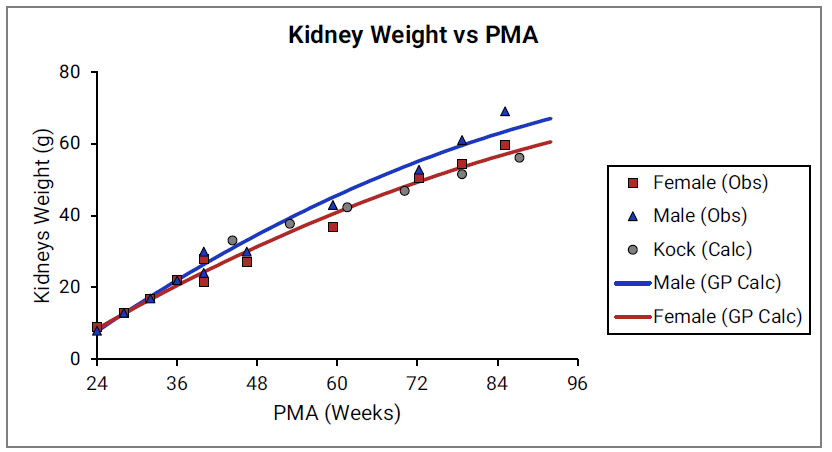
Figure 2-21: Brain weight versus PMA
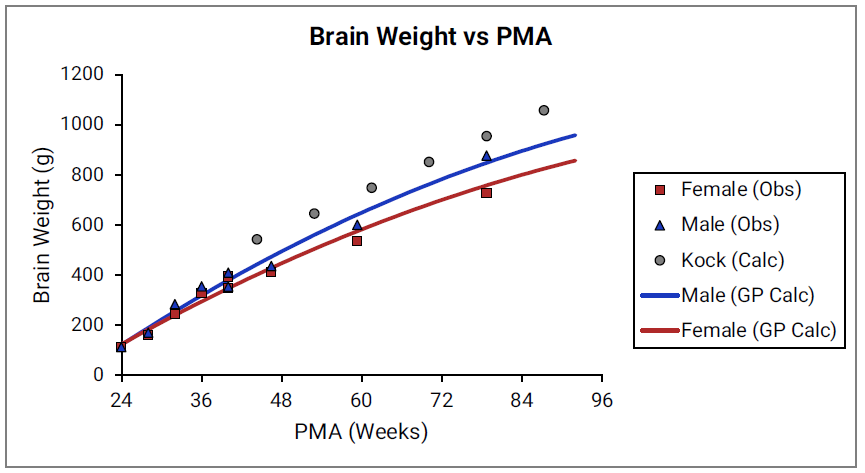
Figure 2-22: Heart weight versus PMA
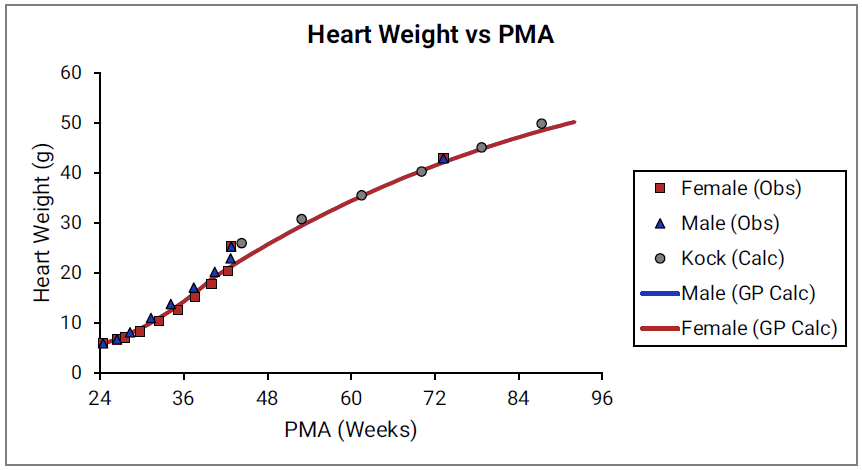
Figure 2-23: Lung weight versus PMA
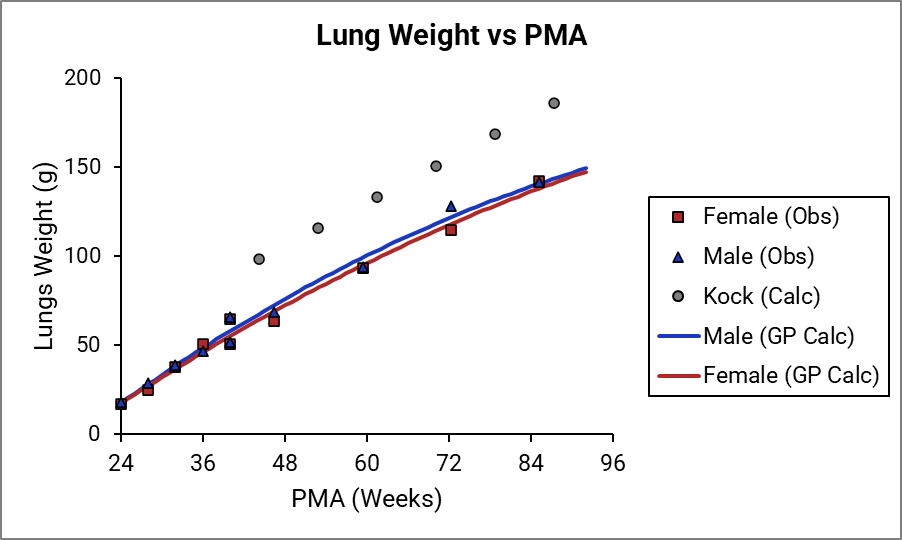
Figure 2-24: Muscle weight versus PMA
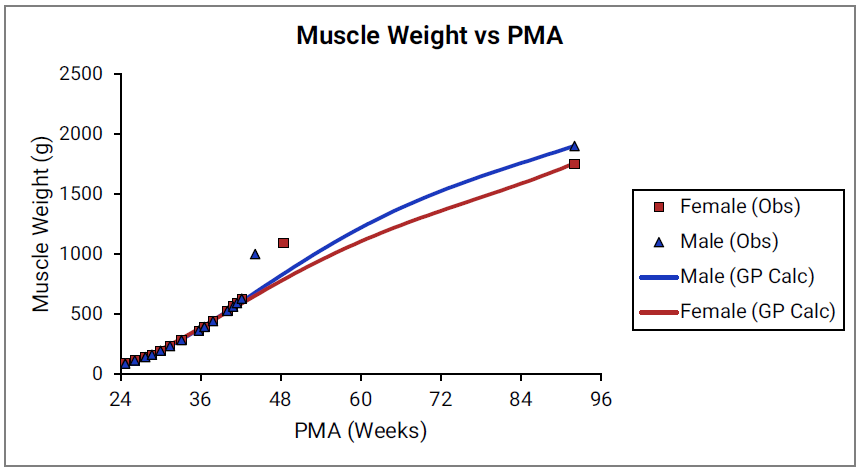
Figure 2-25: Spleen weight versus PMA
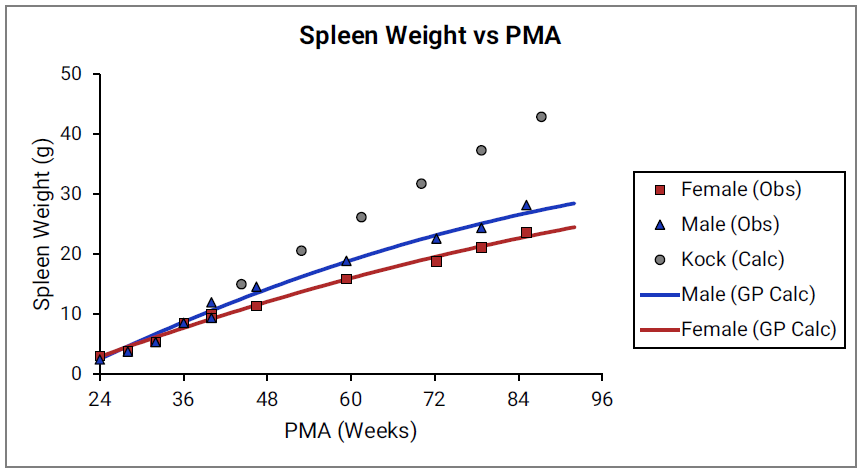
Figure 2-26: Reproductive organ weight versus PMA
Percent fat mass versus post-menstrual age (PMA) for infants up to one year old
Figure 2-27: Term-born infants
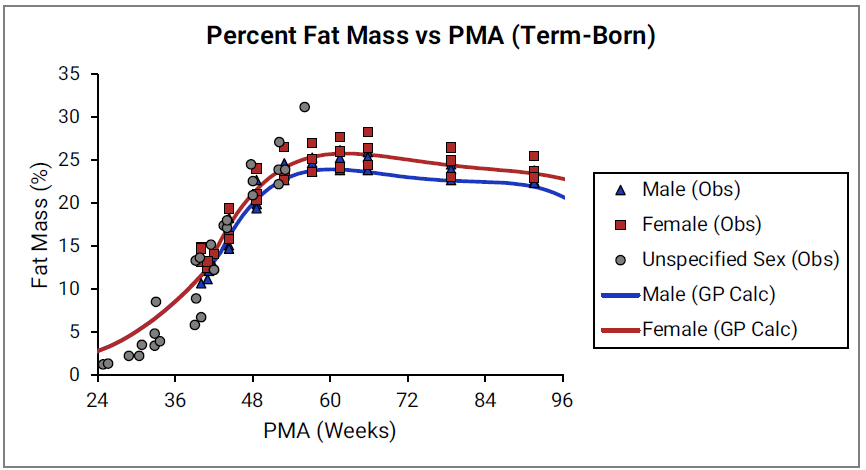
Figure 2-28: Pre-term infants
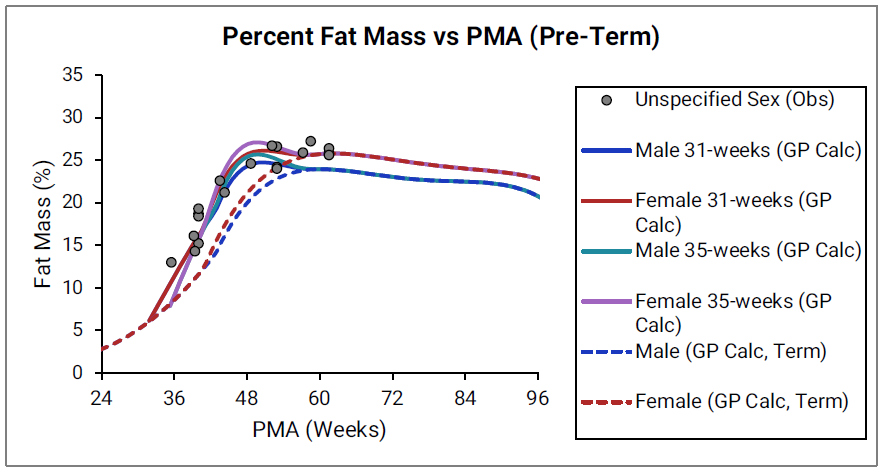
The data points represent experimental data 0 0 0 0 0 0 0 0 0 0 0 0 0 0 . The lines show % fat mass calculated using equations implemented in GastroPlus®. In the top graph, the data for males and females are shown in blue and red, respectively. The data from publications which did not specify the gender are shown in gray. In the bottom graph, the solid and dotted lines show calculated percent fat mass for males (blue) and females (blue). The dotted blue lines and dotted red lines show calculated percent fat mass for term-born infants, which are the same as the blue and red lines for term-born infants in Figure 2-27. The dark blue/ dark red lines and light blue/light red lines represent percent fat mass calculated for infants born after 31 and 35 weeks of gestation, respectively. Gender information was not included in the data for pre-term infants.
Skeletal weight
Figure 2-29: Skeletal weight versus post-menstrual age (PMA) for infants up to two years old
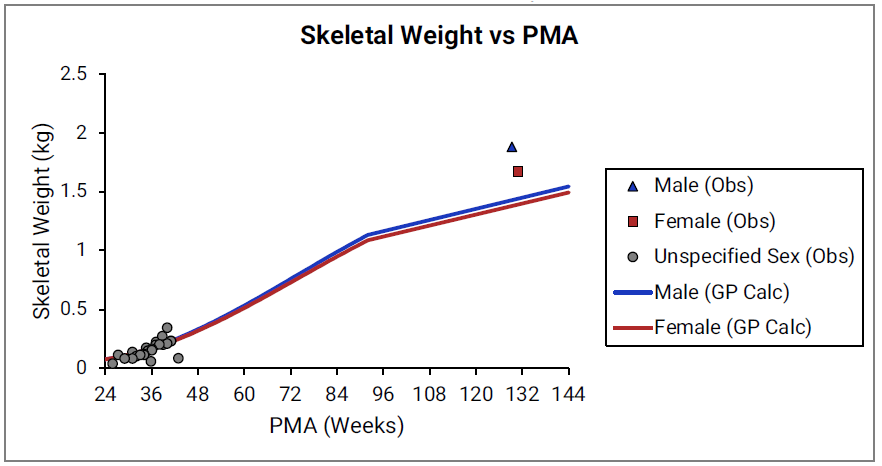
The data points represent experimental data 0 0 0 . The lines show skeletal weights calculated using equations implemented in GastroPlus®.
Figure 2-30: Yellow marrow content shown as fraction of total skeletal weight versus post-menstrual age (PMA) for infants up to one year old
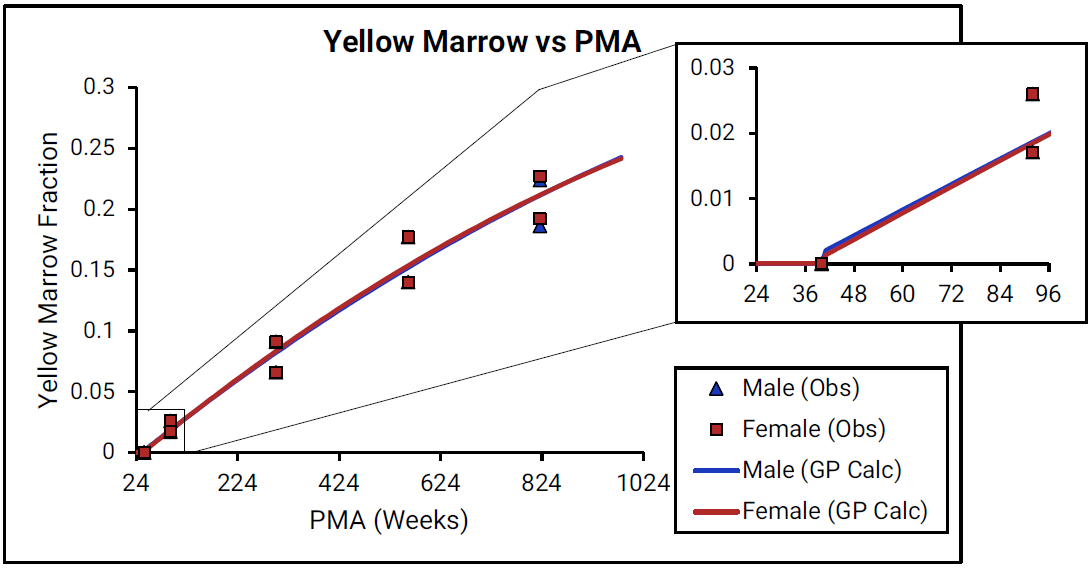
For Figure 2-30, the data points represent experimental data 0 0 . The lines show values that were calculated using equations implemented in GastroPlus®. Data for males and females are shown in blue and red, respectively. As there is very little detailed information for ages from term- born neonates to 1-year-old infants (PMA = 40 to 92 weeks), the insert that is included for each marrow type shows changes in the marrow content from neonates through adulthood (18 years old).
Figure 2-31: Red marrow content shown as fraction of total skeletal weight versus post-menstrual age (PMA) for infants up to one year old
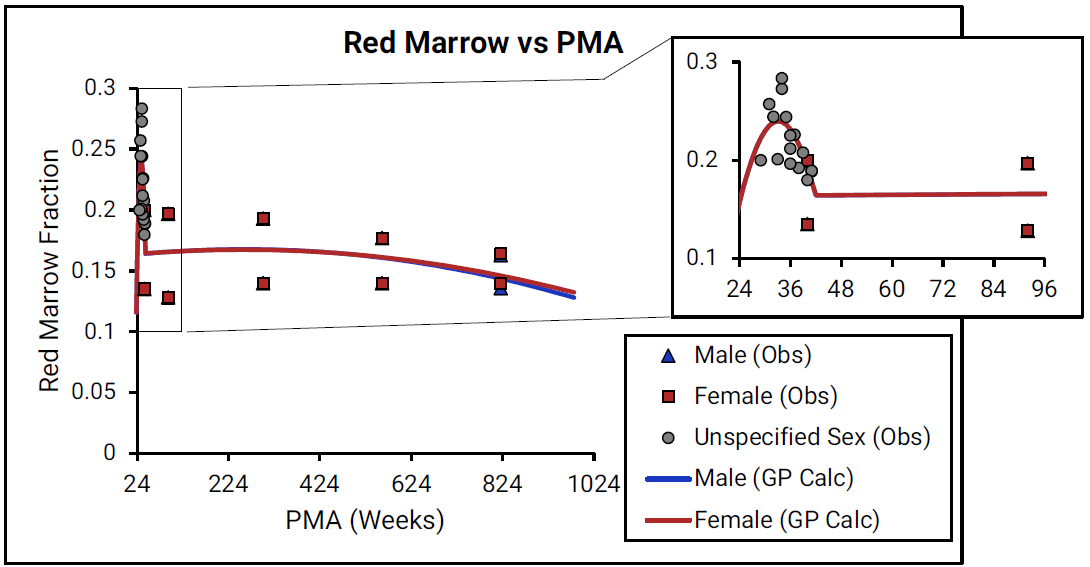
For Figure 2-31, the data points represent experimental data 0 0 . The lines show values that were calculated using equations implemented in GastroPlus®. Data for males and females are shown in blue and red, respectively. As there is very little detailed information for ages from term- born neonates to 1-year-old infants (PMA = 40 to 92 weeks), the insert that is included for each marrow type shows changes in the marrow content from neonates through adulthood (18 years old).
Infant blood parameters
Infant blood parameters include blood volume, cardiac output, drug-binding proteins in plasma, and hematocrit.
Blood volume
Figure 2-32: Blood volume versus post-menstrual age (PMA) for neonates born after 24- 40 weeks of gestation
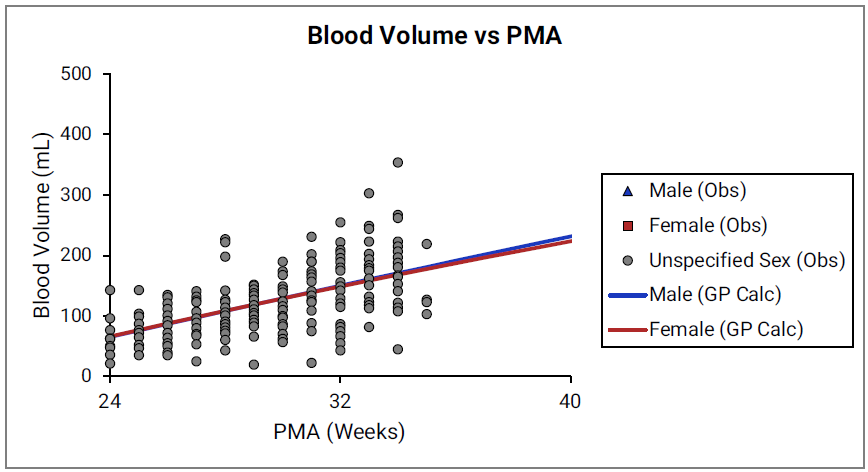
Figure 2-33: Blood volume versus post-menstrual age (PMA) for children up to 3 years old
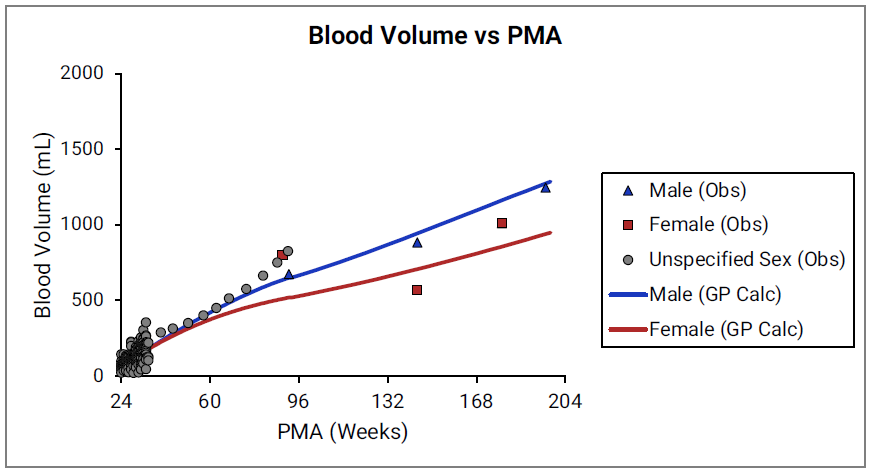
For Figure 2-32 and Figure 2-33, the data points represent experimental data 0 0 0 . The lines show blood volumes calculated using equations implemented in GastroPlus®. The data for males and females are shown in blue and red, respectively. The data from publications that did not specify the gender are shown in gray.
Cardiac output
Figure 2-34: Cardiac output versus post-menstrual age (PMA) for infants up to one year old
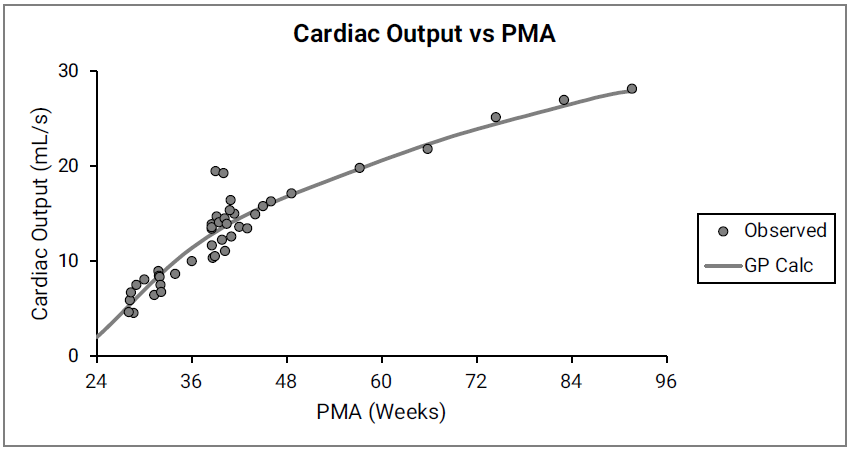
The data points represent experimental data 0 0 0 0 0 0 . The line shows cardiac output calculated in GastroPlus®. Gender information was not included in the data.
Blood proteins and hematocrit
GastroPlus® also accounts for differences in fraction unbound in plasma (fup) and the differences in the blood/plasma drug concentration ratio (Rb:p) between children and adults due to different levels of protein and hematocrit, respectively.
As shown in Equation 2-54, the fup scaling is based on the equation by McNamara 0 and it assumes that the input experimental percent unbound in plasma is representative of non-specific drug to protein binding in adult plasma:
Equation 2-54: McNamara’s equation for infant physiologies
where:
Variable | Definition |
We don't have a way to export this macro. | The fraction of drug unbound in pediatric plasma. |
We don't have a way to export this macro. | The fraction of drug unbound in adult plasma. |
We don't have a way to export this macro. | The protein concentration in pediatric plasma. |
We don't have a way to export this macro. | The protein concentration in adult plasma. |
The ratio of pediatric to adult plasma protein (Cp,protein(ped)/Cp,protein(adult)) is based on the ontogeny of the two major drug-binding proteins in plasma, α1-acid glycoprotein (AAG) and albumin as shown in Figure 2-35 and Figure 2-36.
Figure 2-35: AAG concentration in plasma (shown as % of adult levels) versus post-menstrual age (PMA) for infants up to one year old
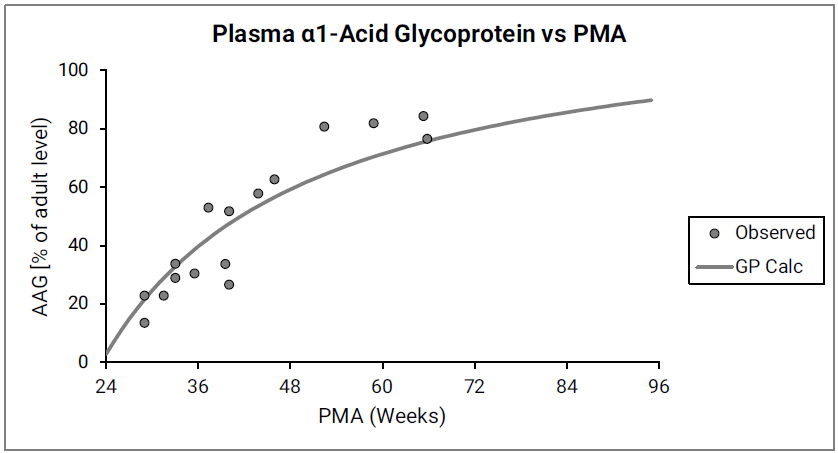
Figure 2-36: Albumin concentration in plasma (shown as % of adult levels) versus post-menstrual age (PMA) for infants up to one year old
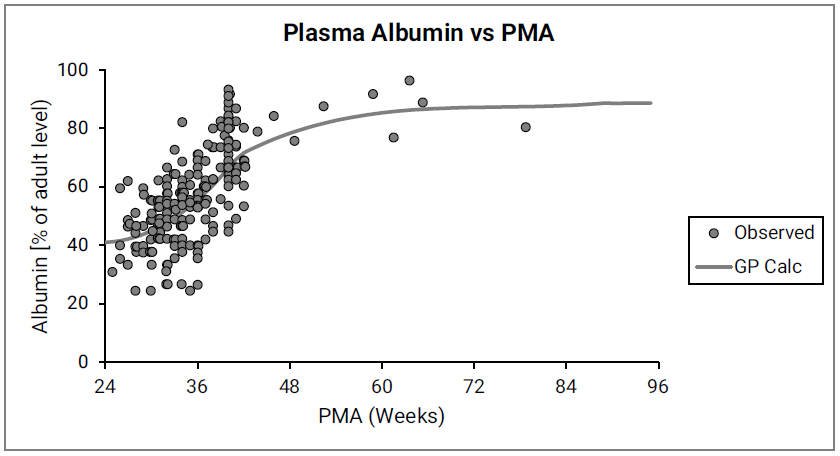
The data points represent experimental data 0 0 0 0 0 0 0 0 0 . The lines show values calculated using equations in GastroPlus®.
McNamara 0 also pointed out the propensity of different compounds to bind preferentially either to plasma AAG or to plasma albumin. Simulations Plus continues to research the structural determinants for this preference, and a more detailed binding model might be implemented in future versions of GastroPlus®. For now, Simulations Plus is combining the ontogeny of AAG and albumin to calculate the changes in total drug-binding plasma protein with age, and then using the total drug-binding protein (AAG + albumin) to scale fup values. Predictions using total drug-binding protein were compared to predictions based on the major binding protein for each compound 0 .
Figure 2-37 summarizes the performance of both approaches.
Figure 2-37: Comparison of predicting infant fup
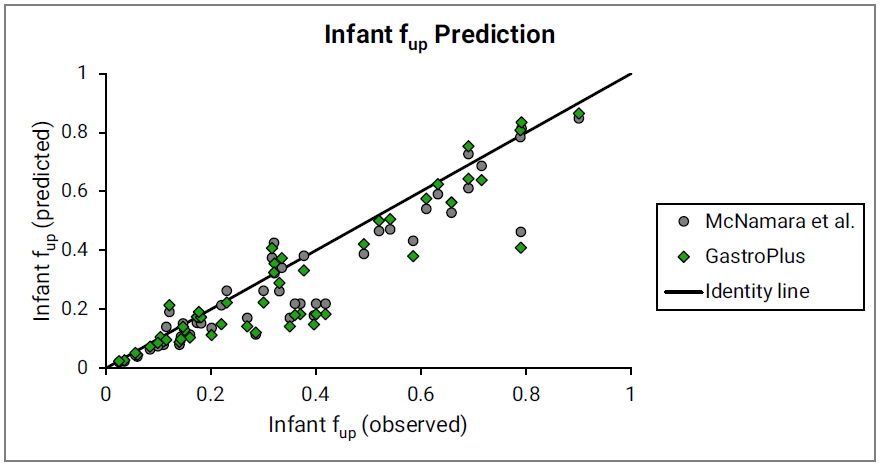
The major binding protein (gray circles) as proposed by McNamara 0 and the total drug-binding protein (green diamonds) as currently implemented in GastroPlus® were used. Observed data are used as compiled by McNamara et al. Identity line is shown for reference.
As shown in Equation 2-55, infant Rb:p scaling uses a derived equation based on the assumption that the input experimental Rb:p value represents binding to red blood cells in adult blood, where the hematocrit = 0.45.
Equation 2-55: Infant Rb:p scaling
where:
Variable | Definition |
We don't have a way to export this macro. | The drug blood/plasma concentration ratio as indicated for infants (ped) or adults (adult). |
We don't have a way to export this macro. | The hematocrit, expressed as a fraction, in pediatric blood. |
We don't have a way to export this macro. | The hematocrit, expressed as a fraction, in adult blood. |
Figure 2-38 shows the changes in hematocrit with age.
Figure 2-38: Hematocrit versus post-menstrual age (PMA) for infants up to 1 year old
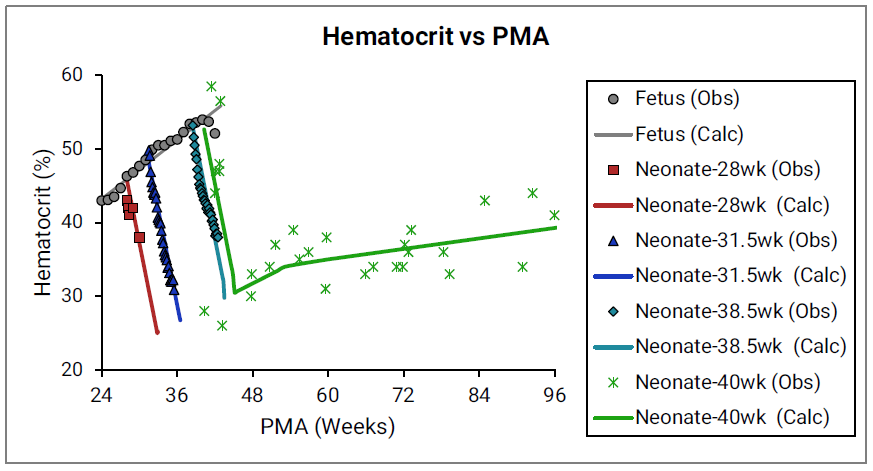
The data points represent experimental data 0 0 0 . The plotted lines represent values calculated using equations in GastroPlus®. Individual colors represent fetus (gray), neonates born after 28 (red), 31.5 (blue), 38.5 (teal), and 40 (green) weeks of gestation. The experimental data shown in green were not accompanied by exact specification of gestation age for each subject 0 , but based on the reported body weights for the youngest subjects and the author’s description of the subjects as “normal well children,” Simulations Plus assumed these subjects to be term-born infants.
Infant tissue compositions
For Figure 2-39 through Figure 2-44, the data points represent experimental data 0 . The plotted lines show values calculated using equations implemented in GastroPlus®. Data for percent of water and total lipid in each tissue are shown in blue and red, respectively. Because only sparse experimental data is available for these tissue compositions, the entire range of ages from newborn born after 24 weeks of gestation up to an 18-year-old adult (PMA = 976 weeks) is shown.
Figure 2-39: Tissue composition versus post-menstrual age (PMA) for adipose
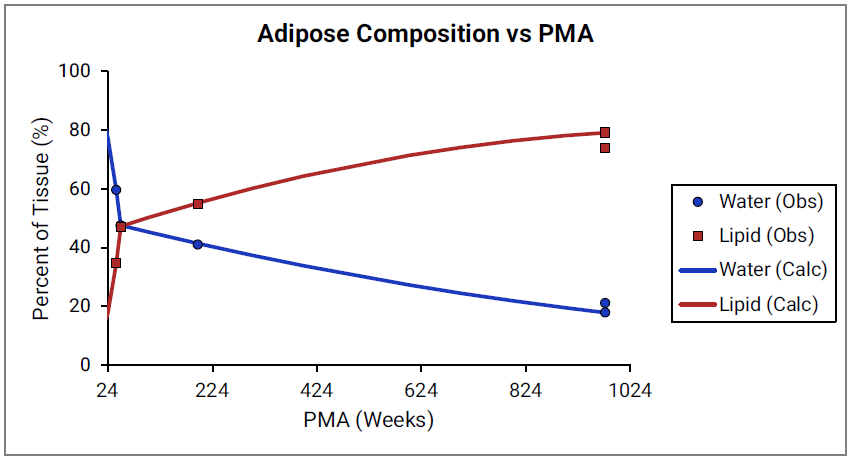
Figure 2-40: Tissue composition versus post-menstrual age (PMA) for brain
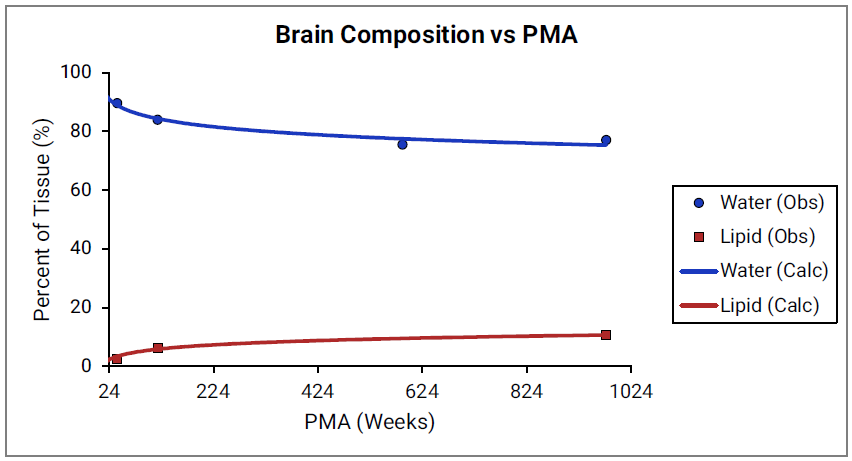
Figure 2-41: Tissue composition versus post-menstrual age (PMA) for kidney
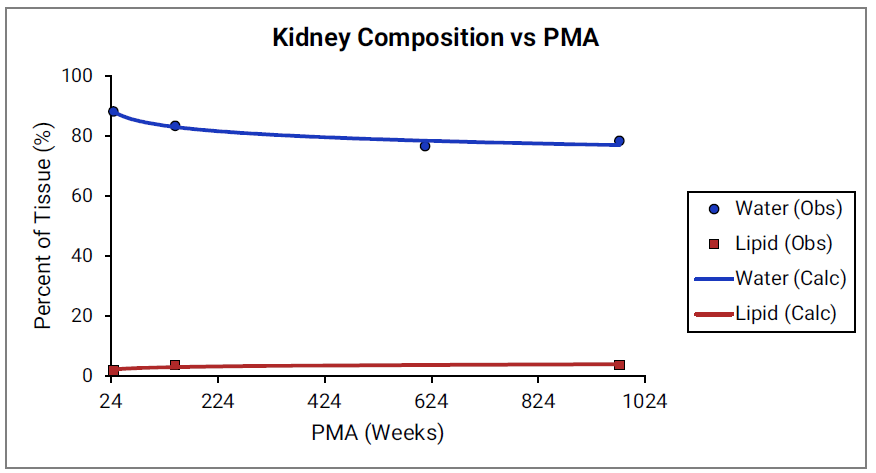
Figure 2-42: Tissue composition versus post-menstrual age (PMA) for lung
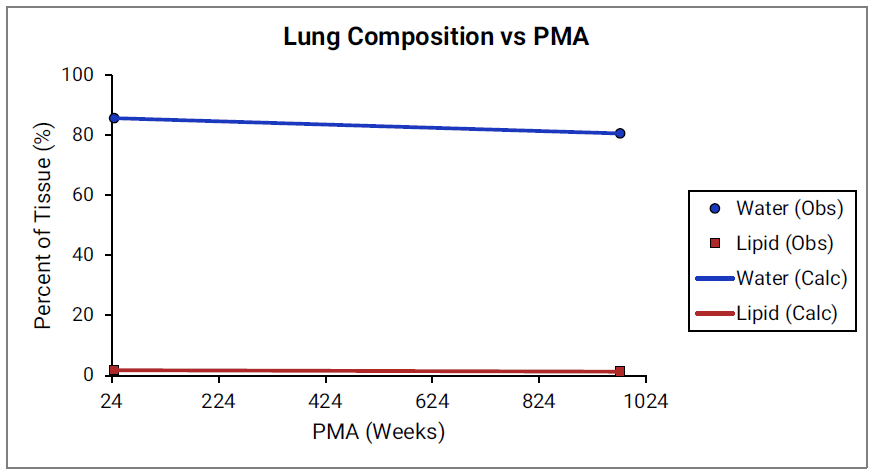
Figure 2-43: Tissue composition versus post-menstrual age (PMA) for heart
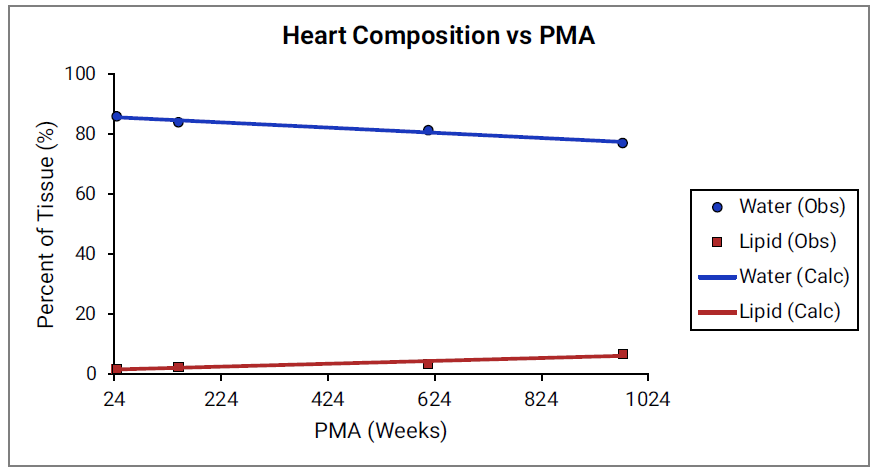
Figure 2-44: Tissue composition versus post-menstrual age (PMA) for liver
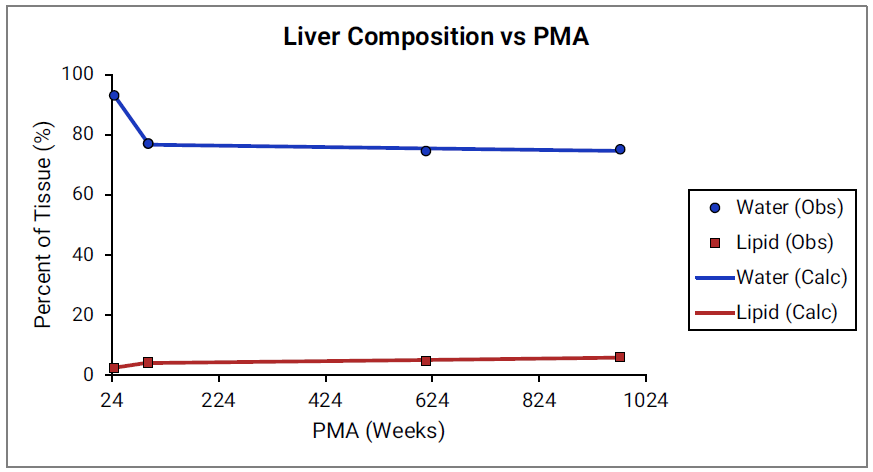
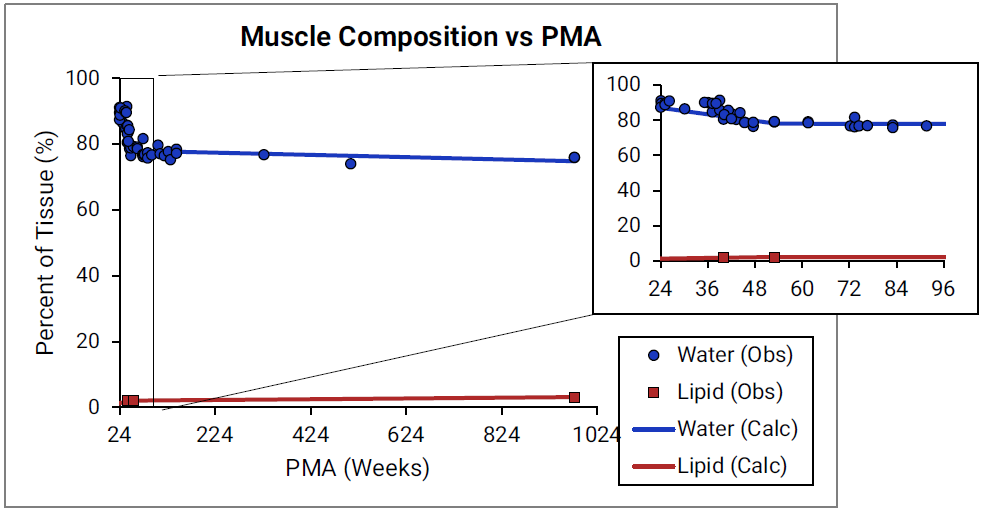
For Figure 2-45 through Figure 2-47, the data points represent experimental data 0 0 0 0 0 . The plotted lines represent values calculated using equations in GastroPlus®. Data for percent of water and total lipid in each tissue are shown in blue and magenta, respectively. Because only sparse experimental data is available for red marrow and spleen tissue compositions, the entire range of ages from newborn born after 24 weeks of gestation up to an 18-year-old adult (PMA = 976 weeks) is shown. Additional rich sets of data for water content in muscle of infants were available in literature 0 0 and an additional plot with details for infants up to 1-year-old is shown as a zoomed in plot for this tissue.
Figure 2-45: Tissue composition versus post-menstrual age (PMA) for red marrow
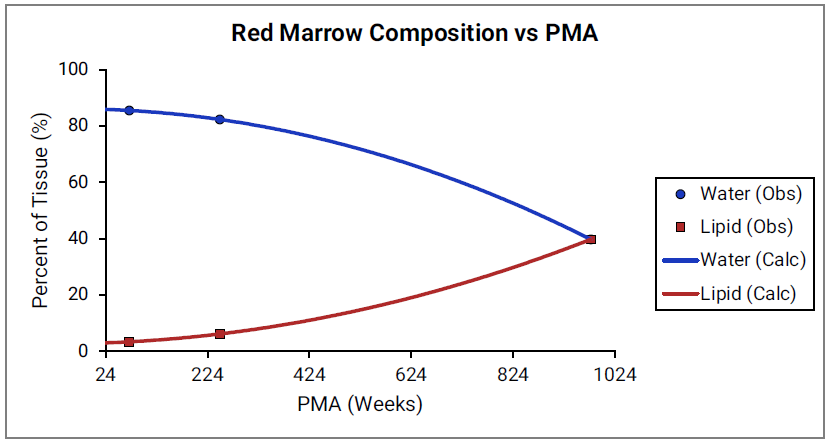
Figure 2-46: Tissue composition versus post-menstrual age (PMA) for spleen
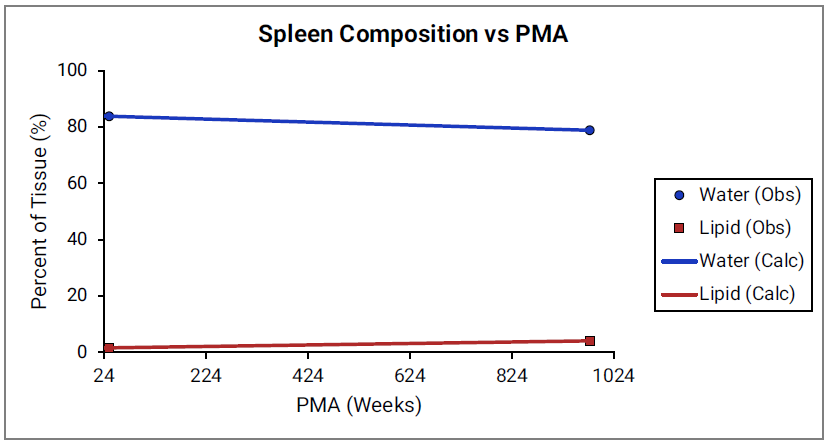
Figure 2-47: Tissue composition versus post-menstrual age (PMA) for muscle
Age dependency for extracellular volume fraction was not found for individual tissues and is estimated from changes in total body water (TBW) and total extracellular water (EXTW).
Figure 2-48: Total body water and extracellular water versus post-menstrual age (PMA)
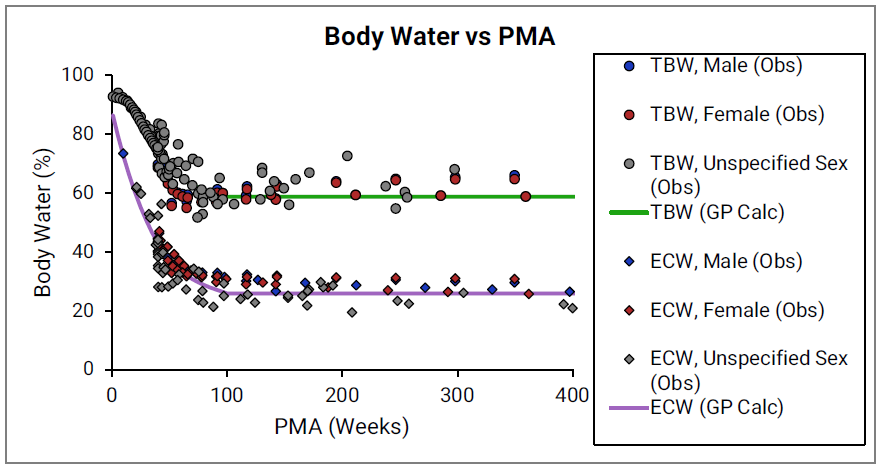
The circles and diamonds represent experimental data for total body water 0 0 0 0 0 and extracellular water 0 0 0 0 0 , respectively. The plotted lines represent values calculated using equations in GastroPlus®. Data for males and females are shown in blue and red, respectively. The data from publications which did not specify the gender are shown in gray.
Infant renal functions
The development of glomerular filtration rate (GFR) and of tubular secretion rate has been the subject of extensive research. Numerous studies have documented changes in GFR in both fetus and post-birth. In general, it was observed that GFR development is slower in utero and exhibits a sharp increase in the first few days after birth or after reaching 33-35 weeks PMA, whichever occurred later. This behavior corresponds to nephrogenesis being completed at approximately 35-36 weeks of gestational age. The equations that are incorporated into GastroPlus® for infant renal functions account for the effects of gestational age and postnatal age on GFR in the first few weeks after birth (Figure 2-49). After approximately 12 weeks of age, the effect of gestational age does not seem to be as dominant, and GFR is estimated based on postnatal age alone (Figure 2-50).
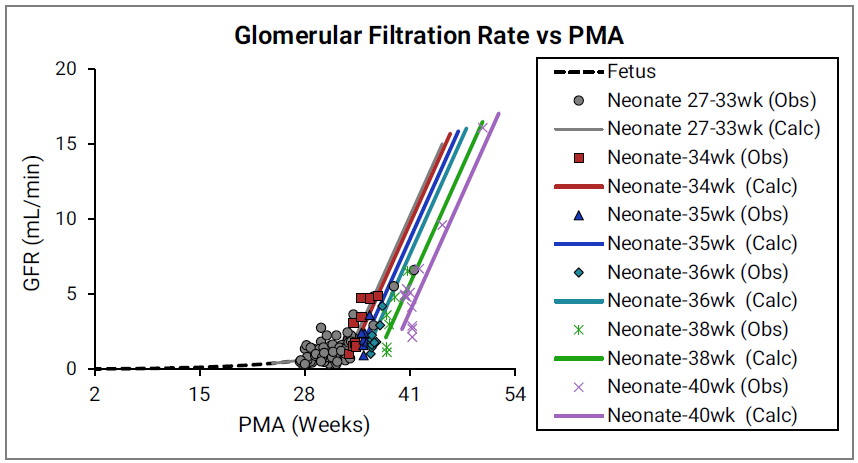
Figure 2-49: GFR versus post-menstrual age (PMA) for neonates up to 12 weeks old (top) and calculated versus observed for same data (bottom)
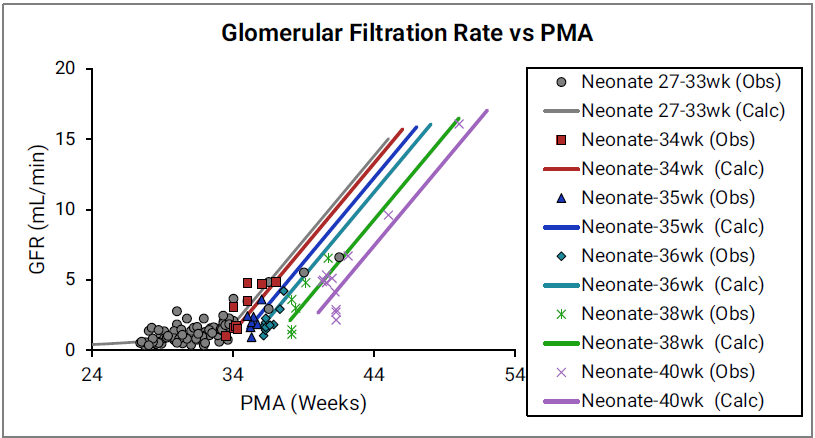
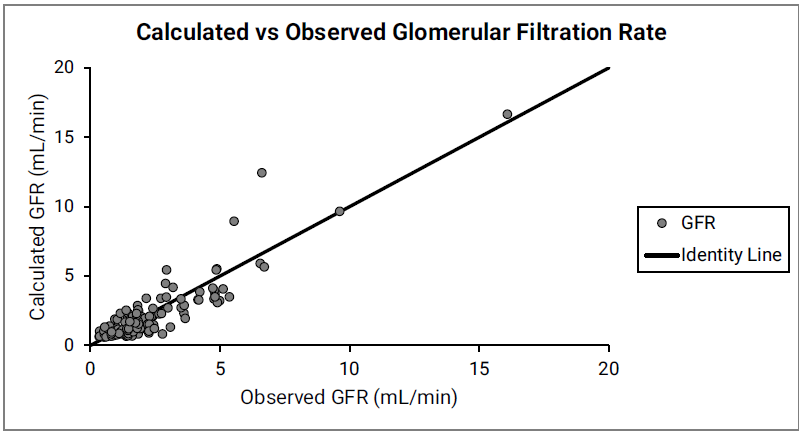
For the top plot, the data points are as follows for the number of weeks born after gestation: 27-33 (gray), 34 (red), 35 (blue), 36 (teal), 38 (green) and 40 (magenta). The data points represent experimental data 0 0 0 0 . Lines show GFR calculated in GastroPlus®.
Figure 2-50: GFR versus post-menstrual age (PMA) for infants and children < 6 years old
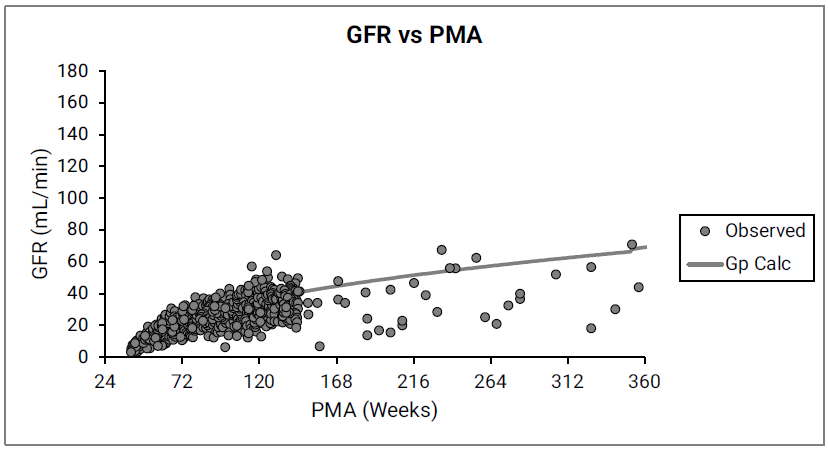
The data points represent experimental data 0 0 0 0 0 . The line shows GFR calculated in GastroPlus® for infants and children from 12 weeks to 6 years of age.
Disease conditions
The PEAR algorithms account for known physiological changes in hepatic impairment, renal impairment and obesity. When creating a new PBPK model, a user has the option to select physiology for different degrees of hepatic or renal impairment. Physiological changes in obesity are accounted for automatically when a user-defined body weight and BMI indicate an obese subject. Brief summaries of the literature sources used to parameterize each of these conditions are provided in the following sections.
Liver cirrhosis
GastroPlus® follows the Child-Pugh classification to characterize different degrees of liver cirrhosis. The systemic physiological changes—functional liver volume, hepatic and intestinal activities of CYP450 enzymes, plasma protein levels, hematocrit, hepatic and arterial blood flow, cardiac output, glomerular filtration rate, and so on—are incorporated as previously published 0 .
Renal Impairment
As summarized in Table 2-6, GastroPlus® follows the classification of renal function in the US FDA guidance (FDA/CDER 2010).
Table 2-6: Classification of renal function in GastroPlus®
Description | eGFR | CLcr |
Normal | >90 | >90 |
Mild decrease in GFR | 60–89 | 60–89 |
Moderate decrease in GFR | 30–59 | 30–59 |
Severe decrease in GFR | 15–29 | 15–29 |
End Stage Renal Disease (ESRD) | <15, not on dialysis | <15, not on dialysis |
The information about systemic physiological changes—GFR, hematocrit, plasma protein levels, hepatic CYP450 enzymes, gastric emptying rates, and so on—was collected from multiple literature sources 0 0 0 0 0 0 0 .
Obesity
As summarized in Table 2-7, GastroPlus® follows the standard WHO definition for overweight and obesity for adults over 18 years old 0 .
https://pubmed.ncbi.nlm.nih.gov/11234459/
Table 2-7: Classification of overweight and obesity for adults by Body Mass Index (BMI)
BMI (kg/m2) | Description | Co-morbidity Risk |
<18.5 | Underweight | Low |
18.5–24.99 | Healthy | Average |
25–29.99 | Overweight | Mildly increased |
30–39.99 | Obese (Combined Obese Class I and Class II) | Moderate to Severe |
>40 | Morbidly obese | Very severe |
As summarized in Table 2-8, GastroPlus® follows the classification suggested by the U.S. Centers for Disease Control and Prevention for overweight and obesity for children between the ages of 2 and 18 years of age. The look-up table that the CDC provides is used to determine the percentiles for each age.
www.cdc.gov/growth-chart-training/hcp/using-bmi/plotting-interpreting-bmi.html
Table 2-8: Classification of overweight and obesity for children (2-18years) by Body Mass Index (BMI)
Description | Percentile Range |
Underweight | <5th percentile |
Healthy | 5th–85th percentile |
Overweight | 85th–95th percentile |
Obese | >95th percentile |
The information about systemic physiological changes—lipid content in muscle, composition of plasma, GFR, tissue sizes as percent of total body weight, hepatic CYP450 enzymes, and so on—was collected from multiple literature sources 0 0 0 0 0 0 0 0 0 0 0 0 0 0 .
Contradicting modifications were suggested in publications for several physiological parameters, such as the GI physiology (transit time, volume, pH values) or UGT expression levels. Until more definitive information can be found, the default values that are used for the parameters are those of healthy (normal weight) subjects.
The changes in tissue sizes as a percent of total body weight are included in the model based on published information 0 0 .
As shown in Figure 2-51, the physiologies also account for variations in the tissue blood flows and the resulting changes in the cardiac output.
Figure 2-51: Cardiac output versus body weight for Caucasian adults
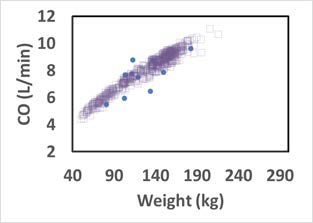
The solid circles represent experimental data 0 . The open squares represent the cardiac output of the virtual subjects (a group of 20 to 60 year old adults) generated by a GastroPlus® algorithm.
Pregnancy
In the PEAR™ module, the Pregnancy model offers two distinct settings based on gestational age (GA):
For a gestational age (GA) between 0-6 weeks, only the uterus tissue is added to the model.
For a GA greater than 6 weeks, in addition to the uterine tissue, placental tissues (fetal and maternal counterparts), amniotic fluid, fetal tissue (as a whole) and fetal (venous return and arterial supply) blood circulation are added to the model.

Because the fetal blood circulation starts to establish itself around five to six weeks of gestation, the gestational age of six weeks was selected to separate the two settings.
Figure 2-52 shows the layout of the GastroPlus® Pregnancy model for a GA greater than six weeks.
Figure 2-52: Layout of the Pregnancy model for a GA greater than six weeks

To view the fetal tissues, double click on Fetal Tissues. To go back to the mother tissues, double click on Mother.
Pregnancy changes modeling
The maternal body incurs a series of physiological changes during pregnancy, including, but not limited to, weight gain, changes in enzyme expression levels, enlargement of certain tissues such as the uterus, placenta, brain and kidney, an increased amount of plasma volume, glomerular filtration rate (GFR) increase, GI changes (increased stomach transit time), and so on 0 0 0 0 0 0 . The volume of fetal tissue and fetal blood also increases with GA. All these changes have been incorporated into the built-in pregnancy PEAR physiology. If you specify the maternal age, GA, and fetal gender, then built-in equations generate the default physiology; however, you can further modify the default physiologies for both maternal and fetal subjects, such as the body weight and height, the cardiac output, maternal weight gain, and individual tissue weights and perfusions.
Maternal weight gain
Weight gain is one of the major changes in the maternal physiology during pregnancy. The total body weight for the maternal subject is the sum of the pre-pregnancy body weight and the weight gain during pregnancy. The pre-pregnancy weight is used to calculate the tissue weights. For tissues which don't change with pregnancy, such as the heart, spleen, liver and lung, the tissue weight stays the same for any GA. For tissues which change with GA, the percent of increase is calculated and added to the pre- pregnancy tissue weight. Carmichael et al. 0 have published weight gain percentiles for different BMI groups. The underweight, normal weight, and overweight subjects have consistent measurements throughout the pregnancy, while the obese group showed significantly lower weight gain compared to the other groups. Similar results were also presented in another study 0 . In GastroPlus®, the averaged weight gain from all underweight, normal weight, and overweight subjects is implemented as shown in Figure 2-53.
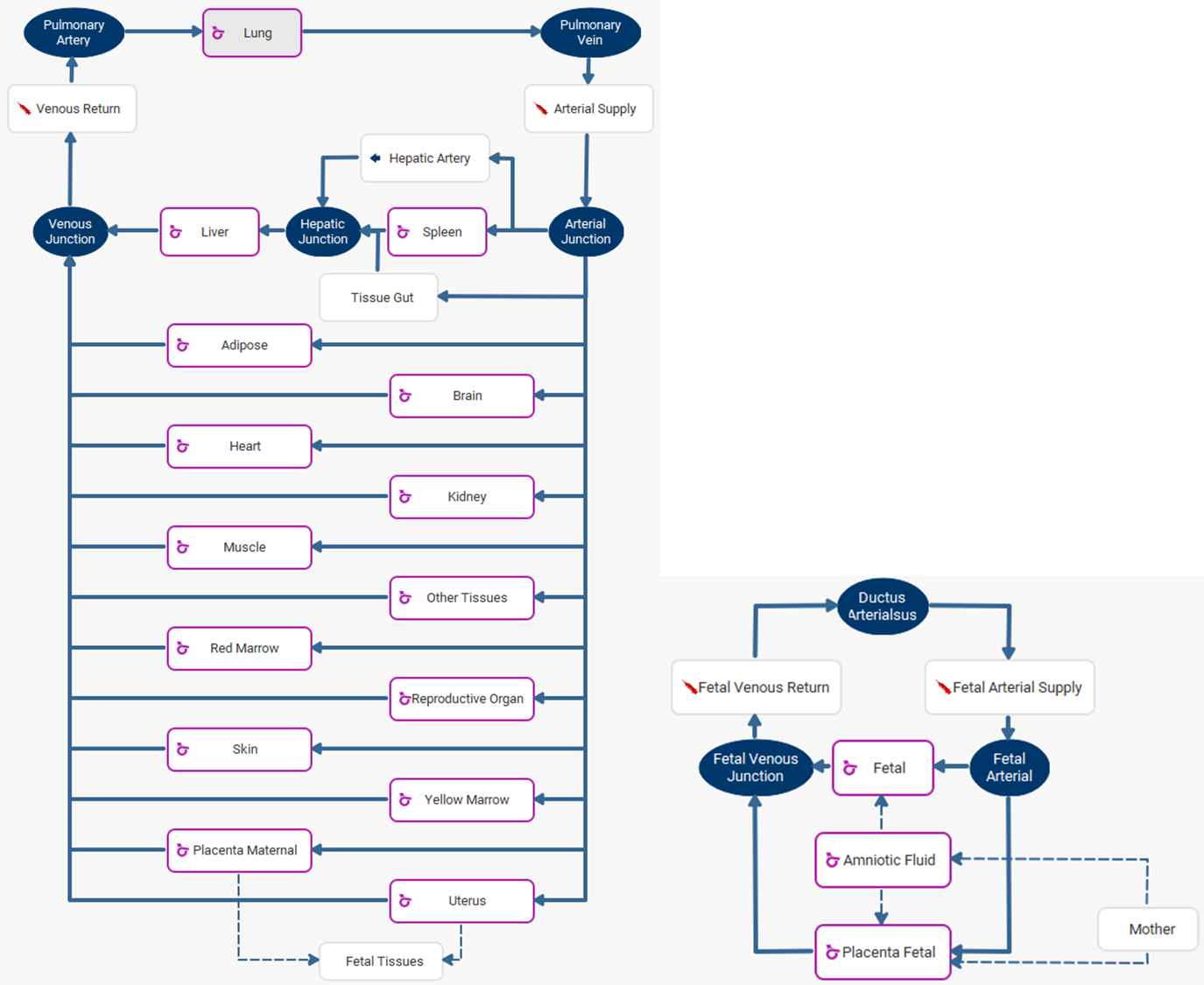
Figure 2-53: Weight gain during pregnancy
The solid red line represents the calculation in GastroPlus®. The color-coded diamonds are the observed weight gain for obese, overweight, normal weight, and underweight subjects 0 .
Fetal height and weight
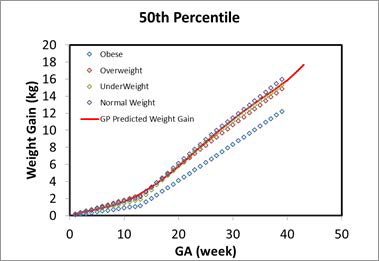
As shown in Figure 2-54, the fetal weight and height at early pregnancy were extrapolated from the preterm (12-16 weeks) infant and fetus measurements, which is the same data that was used for developing the infant PBPK models. The other measurements, such as GFR, fetal hematocrit, liver size (which is used in the scaling for enzyme expressions), blood volume, and percent of protein as adult level, were either extrapolated from current infant equations or refitted against the available data for early pregnancy. Figure 2-55 shows the hematocrit versus either the PMA for infants up to a year old or the GA for a fetus which was refitted with the Dallmann 0 equation and available observed data which were used to build the infant PBPK models. Figure 2-54 shows the GFR versus either the PMA for infants up to a year old or the GA for a fetus, which was extrapolated from infant PBPK models.
Figure 2-54: Fetal weight and height prediction in GastroPlus® versus observed data
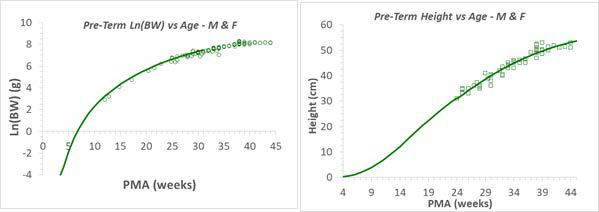
The solid line in each graph represents the GastroPlus® predicted value for fetal weights (left) and heights (right). The green circles and squares are the observed values from multiple publications. Refer to Infant physiologies for the referenced publications.
Figure 2-55: Hematocrit versus post-menstrual age (PMA) for infants up to 1 year old or gestational age (GA) for fetus
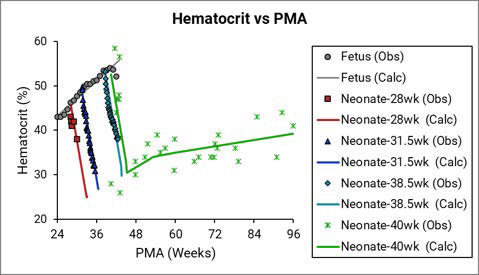
The legend is identical to that for Figure 2-38. The solid yellow triangles represent data that was calculated from the equation that was provided in the Dallmann et al. reference 0 . The final hematocrit equation (the black line) for a fetus was fitted with the Dallmann equation and the rest of the observed data.
Figure 2-56: GFR versus post-menstrual age (PMA) for infants up to 1 year old or gestational age (GA) for a fetus
The legend is identical to that for Figure 2-49. The earliest observed values were measured for pre-term infants at 28 weeks. Simulations Plus extrapolated the curve to calculate the GFR for a fetus less than 28 weeks.
Pregnancy pathways modeling 0
Amniotic fluid is a clear, yellowish liquid that surrounds the fetus during pregnancy. This fluid is crucial in fetal development. It provides necessary nutrients such as electrolytes, liquids, carbohydrates, and proteins while serving as a barrier for bacteria growth and absorbing outside pressure and physical forces. Maintaining the normal amount of fluid ensures the fetus development. Conditions such as oligohydramnios, which is too little amniotic fluid), or polyhydramnios, which is too much amniotic fluid, can cause fetal growth disorder and preterm labor. The regulation of the amniotic fluid includes three pathways 0 :
The trans-membrane pathway: The movement of water and solute between amniotic fluid and maternal blood within the wall of the uterus.
The intra-membranous pathway: The transfer of water and solutes from the amniotic cavity to the fetal circulation (blood) across the amniotic membranes. This transfer occurs in the placenta, the fetal skin, and the umbilical cord. The pathway can be divided into active and passive components, with vesicular pressure driving the active component and osmosity driving the passive component.

The intra-membranous pathway partially disappears after the fetus skin is fraternized at a gestational age (GA) = 20 weeks.
The fetal pathway: The transfer of water and solutes between the amniotic fluid and fetal tissue.
These three pathways have been simplified and incorporated into GastroPlus®.
Fetal and amniotic fluid tissues modeling
The rate constants for the movement of the water and solute are not well-established for a human. Some measurements are available for ovine fetus 0 0 , which have been adapted by scaling based on the fetal body weight. Equation 2-56 describes the homeostasis of amniotic fluid.
Equation 2-56: Homeostasis of amniotic fluid
where:
Variable | Definition |
We don't have a way to export this macro. | The fetal urinary rate. |
We don't have a way to export this macro. | The secretion rate from fetal tissue. |
We don't have a way to export this macro. | The fetal swallowing rate. |
We don't have a way to export this macro. | The active portion of the intra-membranous pathway. |
Perfusion-limited fetal tissues
Because the amniotic fluid is surrounded by amnion and is avascular in humans 0 , the tissue type of the amniotic compartment is different from other tissues. For convenience of calculating the model derivatives, the tissue type of the amniotic compartment in GastroPlus® has been set to the same tissue type as that of the fetal compartment, which results in the fetal tissue being modeled as a whole tissue in GastroPlus®. During modeling, you have the option of selecting a balanced rates option, which automatically balances the in and out rates for the amniotic fluid based on the homeostasis assumption. You also have the option of specifying a Rate Scale Factor, which simultaneously scales the following five rate constants:
Variable | Definition |
We don't have a way to export this macro. | The secretion rate from fetal tissue. |
We don't have a way to export this macro. | The fetal swallowing rate. |
We don't have a way to export this macro. | The intramembranous fluid transfer rate constant (defined in the amniotic fluid compartment) that represents osmosis and vesicular routes, respectively. |
We don't have a way to export this macro. | The transmembranous fluid transfer rate constant (defined in the amniotic fluid compartment) that between the amniotic fluid and the uterus. |
Equation 2-57 and Equation 2-58 detail the fetal pathways, which are modeled directly as interactions between the fetal tissue and the amniotic fluid. For perfusion-limited tissue, the solute exchange happens between the amniotic fluid and the vascular space of the fetal tissue.
Equation 2-57: Fetal pathway for perfusion-limited tissue (amniotic fluid)
where:
Variable | Definition |
We don't have a way to export this macro. | The total volume of the amniotic fluid. |
We don't have a way to export this macro. | The total concentration of drug in the amniotic fluid. |
We don't have a way to export this macro. | The concentration of unbound drug in the amniotic fluid. |
We don't have a way to export this macro. | The total concentration of drug in the fetal tissue. |
We don't have a way to export this macro. | The intra-membranous fluid transfer rate constant (defined in the amniotic fluid compartment) representing osmosis. |
We don't have a way to export this macro. | The trans-membranous fluid transfer rate constant between the uterus and the amniotic fluid. |
We don't have a way to export this macro. | The fetal tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The fraction of unbound drug in the fetal plasma. |
We don't have a way to export this macro. | The total concentration of drug on the fetal side of the placenta. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient on the fetal side of the placenta. |
We don't have a way to export this macro. | The fetal filtration clearance rate. |
We don't have a way to export this macro. | The secretion rate from fetal tissue. |
We don't have a way to export this macro. | The fetal swallowing rate. |
We don't have a way to export this macro. | The total concentration of drug in the uterine tissue. |
We don't have a way to export this macro. | The uterine tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The general clearance rate for the fetal tissue. |
Unless otherwise noted, all variables are defined for the amniotic fluid or fetal tissue.
Equation 2-58: Fetal pathway for perfusion-limited tissue (fetal tissue)
where:
Variable | Definition |
We don't have a way to export this macro. | The total volume of the fetal tissue. |
We don't have a way to export this macro. | The fetal blood flow. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood in fetal tissue. |
We don't have a way to export this macro. | The fetal blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The fetal tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The intra-membranous fluid transfer rate constant (defined in the amniotic fluid compartment) representing osmosis and vesicular routes, respectively. |
We don't have a way to export this macro. | The concentration of unbound drug in the amniotic fluid. |
We don't have a way to export this macro. | The total concentration of drug in the fetal tissue. |
We don't have a way to export this macro. | The fraction of unbound drug in the fetal plasma. |
We don't have a way to export this macro. | The fetal filtration clearance rate. |
We don't have a way to export this macro. | The secretion rate from fetal tissue. |
We don't have a way to export this macro. | The fetal swallowing rate. |
We don't have a way to export this macro. | The general clearance rate for the fetal tissue. |
Permeability-limited fetal tissues
For permeability-limited tissue, the solute exchange happens between the amniotic fluid and the extravascular space of the fetal tissue. Consequently, to model the fetal pathway for permeability-limited tissue, the fetal tissue concentration in Equation 2-57 and in Equation 2-58 is automatically replaced with the fetal extravascular concentration.
Placenta tissue modeling
The placenta tissue is modeled as two compartments: maternal placenta and fetal placenta, where the maternal placenta is facing the maternal blood circulation and the fetal placenta is facing the fetal blood circulation. The two compartments represent the same tissue, and therefore, both compartments must be modeled as the same tissue type: either both perfusion limited or both permeability limited.
For the equations that describe the modeling of the fetal and maternal placenta tissue, the variables were defined according to the following rules:
Variable | Definition |
We don't have a way to export this macro. | Represents concentration, amount (mass) and volume, respectively. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in tissue. |
We don't have a way to export this macro. | Represents fetal, maternal, and total (across both fetal and maternal), respectively. |
We don't have a way to export this macro. | The extracellular fluid to plasma partition coefficient. |
We don't have a way to export this macro. | The (permeability*surface area) product for the tissue, which can be either on the apical side (api) or the basolateral side (baso). |
We don't have a way to export this macro. | The tissue/plasma partition coefficient between maternal tissue and maternal plasma. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient between fetal tissue and maternal plasma. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient between fetal tissue and fetal plasma. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient between total placenta tissue (maternal and fetal) and maternal plasma. |
We don't have a way to export this macro. | The total amount (mass) of drug in the maternal placenta and the fetal placenta, respectively. |
We don't have a way to export this macro. | The maternal blood/plasma drug concentration ratio and the fetal blood/plasma concentration ratio, respectively. |
We don't have a way to export this macro. | The tissue blood flow from the maternal side and the fetal side, respectively. |
We don't have a way to export this macro. | The total maternal placenta tissue volume and the total fetal placenta tissue volume, respectively. |
We don't have a way to export this macro. | Represents total, unbound, extracellular space, intracellular space, and the plasma compartment in the tissue, respectively. |
Perfusion-limited placenta tissues
Figure 2-57 shows the redistribution of drug in perfusion-limited placenta. This redistribution is calculated instantaneously between the maternal placenta tissues and the fetal placenta tissues at any given time.
Figure 2-57: Perfusion-limited placenta tissues
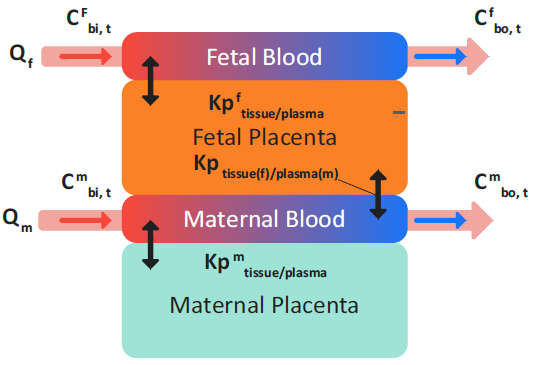
Equation 2-59 (an equation set) defines the total mass balance in a perfusion- limited placenta tissue without clearance and considers only instantaneous distribution that is parameterized by Kp values.
Equation 2-59: Mass balance in perfusion-limited placenta tissue
Therefore, the total concentration in the placenta (maternal + fetal) can be calculated as shown in Equation 2-60.
Equation 2-60: Total concentration in the placenta (maternal + fetal)
Equation 2-61 shows the calculation for the tissue/plasma partition coefficient between the total placenta tissue (maternal + fetal) and the maternal plasma.
Equation 2-61: Partition coefficient for total placenta tissue and maternal plasma
By substituting Equation 2-60 and Equation 2-61 into Equation 2-59, the general equation (Equation 2-62) that describes the change in the amount of drug in the total perfusion-limited placenta tissue (maternal placenta + fetal tissue) is derived. In this equation, the clearance term is the combination of any intrinsic and enzymatic clearance in the placenta tissue and the transflux term represents the drug transport between the amniotic fluid and the fetal placenta through the intra-membranous osmosis pathways.
Equation 2-62: General equation that describes the change in the amount of drug in the total perfusion-limited placenta tissue (maternal placenta + fetal tissue)
where:
Variable | Definition |
We don't have a way to export this macro. | The concentration of unbound drug in the amniotic fluid. |
We don't have a way to export this macro. | The total concentration of drug in the fetal side of the placenta. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient on the fetal side of the placenta. |
We don't have a way to export this macro. | The fraction of unbound drug in the fetal tissue. |
As shown in Equation 2-63, (an equation set), the general equation for the change in the amount of drug in the total placenta can then be used to obtain the change in the amount of drug in the fetal placenta.
Equation 2-63: Change in the amount of drug in the fetal placenta
And, as shown in Equation 2-64, the general equation for the change in the amount of drug in the total placenta can also be used to obtain the initial equation for the change in the amount of drug in the maternal placenta.
Equation 2-64: Initial equation for the change in the amount of drug in maternal placenta
By substituting the fetal tissue placenta concentration (Equation 2-65) into Equation 2-64, the final equation (Equation 2-66) for the change in the amount of drug in the perfusion-limited maternal placenta is derived.
Equation 2-65: Fetal tissue placenta concentration
Equation 2-66: Final equation for the change in the amount of drug in the maternal placenta
Permeability-limited placenta tissue
If the placenta is modeled as permeability-limited tissue, then as shown in Figure 2-58, transplacental transfer is enabled, which represents the interchange between the maternal blood plasma in the intervillous space and fetal trophoblastic cells.
Figure 2-58: Permeability-limited placenta tissue
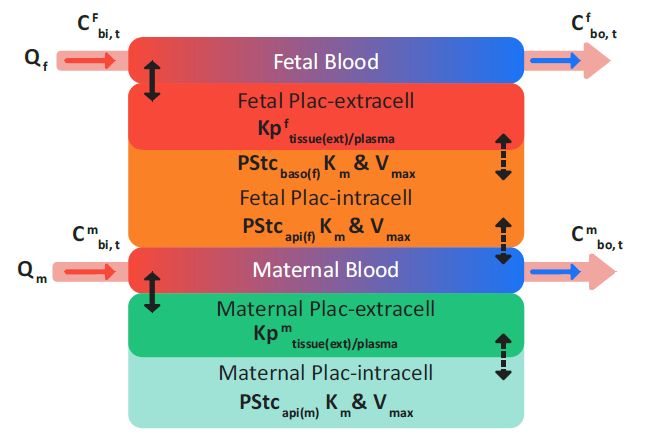
where:
Variable | Definition |
We don't have a way to export this macro. | The permeation between the fetal placenta tissue and the maternal vascular space. Note: The Papi calculation shown in Equation 2-67 and Equation 2-68 is for the same process: Permeation between the fetal placenta tissue and the maternal vascular space. |
We don't have a way to export this macro. | The permeation between the tissue intracellular space and the extracellular space in fetal or maternal placenta tissue. |
We don't have a way to export this macro. | The drug permeation between the amniotic fluid and the fetal placenta tissue as defined by the trans-membranous transport rate, ktransm. |
Equation 2-67: Permeability-limited fetal placenta calculations
Equation 2-68: Permeability-limited maternal placenta calculations
where:
Variable | Definition |
We don't have a way to export this macro. | The saturable clearance rate of drug calculated using Michaelis-Menten kinetics. |
We don't have a way to export this macro. | The saturable, carrier-mediated transport rate of drug into the tissue using Michaelis-Menten kinetics. |
We don't have a way to export this macro. | The saturable, carrier-mediated transport rate of drug out of the tissue using Michaelis-Menten kinetics. |
Enzyme phenotypes
The PEAR algorithms in GastroPlus® account for genetic variants of CYP2B6, 2C9, 2C19, and 2D6 enzymes in Caucasian and Asian subjects. The common genotypes of these CYP enzymes were assigned to poor metabolizer (PM), intermediate metabolizer (IM), extensive metabolizer (EM) or ultra-rapid metabolizer (UM) phenotypes in both populations.
Table 2-9: Enzyme Phenotypes and Genotypes for PEAR algorithms
Enzyme | Phenotype | Genotype |
2C9 | EM | *1/*1 |
IM | *1/*2; *1/*3 | |
PM | *2/*2; *2/*3; *3/*3 | |
2C19 | UM | *1/*17; *17/*17; |
EM | *1/*1 | |
IM | *1/*2; *1/*3; *1/*5; *1/*8; *2/*17 | |
PM | *2/*2; *2/*3; *2/*5; *2/*6; *3/*3 | |
2D6 | UM | Duplicate EM alleles |
EM | *1; *2; *2A; *35 | |
IM | *9; *10; *17; *29; *41 | |
PM | *3; *4; *5; *6; *7; *8; *14; *36; *71 | |
2B6 | EM | *1/*1; *1/*4; *1/*6 |
PM | *5/*5; *5/*6; *6/*6 |
The default frequencies of different phenotypes in Caucasian and Asian (Chinese and Japanese) subjects that are included in GastroPlus® are based on extensive collection of physiological and genetic information from multiple literature resources 0 0 0 0 0 0 0 0 0 0 .
In GastroPlus®, the different phenotypes are assumed to be expressed through variations in the hepatic enzyme abundance. When creating a new PBPK model, the expression for different enzyme phenotypes with the suffix as “-UM,” “-EM,” “IM,” and “PM” are generated based on the selected population and other information (for example. gender, age, and health status) that you provide. A user can set the phenotype of any enzyme for a given subject by selecting an enzyme name with the correct phenotype suffix. Assuming that the percent of expression differences are the same for both liver and gut, the corresponding enzyme expression in gut is also adjusted based on the change in the hepatic abundance due to the phenotype difference.
