Saturable Metabolism in the GI Tract and Liver
After oral absorption, saturable metabolism potentially occurs in the GI tract, and then in the liver. See:
Saturable GI tract metabolism
Figure 1-8 is a representation of the different competing linear and non-linear absorption processes in the GI tract that participate in drug transfer from the lumen to the bloodstream. Drug transfer across the apical membrane can be active or passive, or both. Saturable GI tract metabolism, saturable GI tract efflux, and basolateral transport are competing processes that occur in the enterocytes.
Figure 1-8: Competing linear and non-linear absorption processes in the GI tract
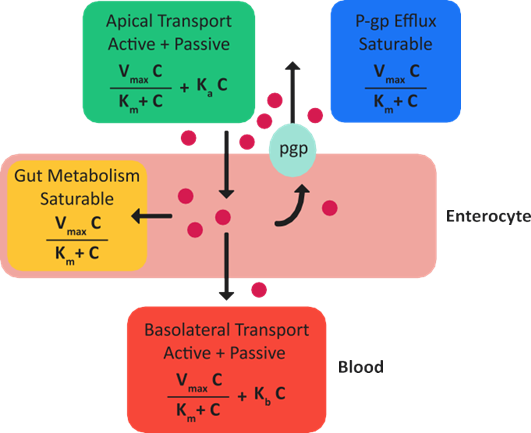
GI tract metabolism is assumed to follow Michaelis-Menten kinetics. Equation 1-61 is the calculation for the total rate of GI tract metabolism in all GI enterocyte compartments (index j) due to all enzymes (index i).
Equation 1-61: Saturable GI tract metabolism rate
where:
Variable | Definition |
We don't have a way to export this macro. | The index for the ith gut metabolism enzyme. |
We don't have a way to export this macro. | The index for the jth enterocyte compartment. |
We don't have a way to export this macro. | The concentration of unbound drug in the enterocyte sub-compartment of the jth intestinal compartment. |
We don't have a way to export this macro. | The fraction of unbound drug in the enterocytes. |
We don't have a way to export this macro. | The maximum velocity of the ith enzyme. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith enzyme. |
We don't have a way to export this macro. | The scale factor for the maximum velocity for the ith enzyme in the enterocyte sub-compartment of the jth intestinal compartment. |
We don't have a way to export this macro. | The scale factor for overall enzyme velocity. The default value is 1.0. |
We don't have a way to export this macro. | The scale factor for overall Km for gut enzymes. The default value is 1.0. |
Only the free drug concentration in the enterocyte is available for GI extraction. If the enzyme is also present in the liver and if Vmax and Km data are available only from liver microsomes (as is typically the case), then you can use the same Vmax and Km for the GI tract, as long as the regional distribution of enzymes in the GI tract is expressed as an amount relative to the whole liver amount of the same enzyme. This is the method used for the GI tract enzyme distribution factors where the numbers represent the following ratio:
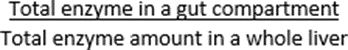
where the typical default value for liver weight is 1800g.
Saturable liver metabolism
Traditional linear predictions of hepatic clearance using the venous equilibrium model for the liver make one or more of the following assumptions:
No drug accumulates in the liver, that is, the liver behaves as a reactor at steady state.
Extraction proceeds in the linear regime of Michaelis-Menten kinetics, that is, the intrinsic clearance is independent of concentration.
Binding of drugs to erythrocytes does not affect hepatic clearance.
GastroPlus® includes a physiologically-based, non-steady-state, non-linear hepatic extraction model that you can use if you suspect non-linear hepatic clearance. In this model, the liver is treated as a separate extraction compartment, perfused by the hepatic blood flow. Before absorbed drug can become systemically available, it must pass from the portal vein through the liver. Systemic drug is also extracted during passage through the liver. Hepatic blood flow and the apparent volume of the liver control the residence time of drug in the liver. Figure 1-9 is a schematic of the GastroPlus® Hepatic Extraction model.
Figure 1-9: GastroPlus® Hepatic Extraction model
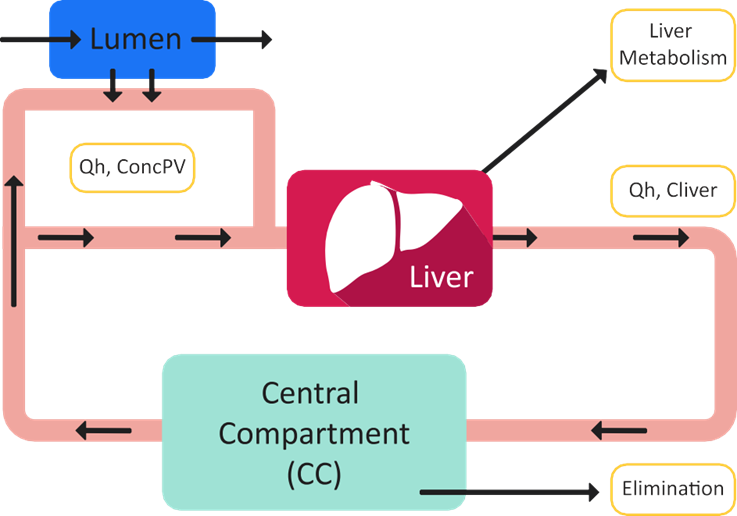
In GastroPlus®, the liver is assumed to behave as a well-stirred tank, which means that extraction occurs at the exit drug concentration. Equation 1-62 is the differential equation for liver extraction.
Equation 1-62: GastroPlus® Hepatic Extraction model, differential equation
where:
Variable | Definition |
We don't have a way to export this macro. | The hepatic flow rate. |
We don't have a way to export this macro. | The whole blood to plasma drug concentration ratio. |
We don't have a way to export this macro. | The total drug concentration in the portal vein. |
We don't have a way to export this macro. | The total drug concentration in the liver. |
We don't have a way to export this macro. | The rate of metabolism, which is calculated separately for each liver enzyme at each time point, and then the total rate of metabolism is calculated as the sum of all the rates of metabolism for all the individual enzymes according to Equation 1-63. |
Equation 1-63: Sum total rate of metabolism for all liver enzymes
where:
Variable | Definition |
We don't have a way to export this macro. | The index for the ith enzyme. |
We don't have a way to export this macro. | The plasma concentration of the drug in the liver. |
We don't have a way to export this macro. | The fraction of unbound of the drug in plasma. |
We don't have a way to export this macro. | The maximum metabolism rate for the ith enzyme. |
We don't have a way to export this macro. | The Michael-Menten constant, which is the concentration at 1/2 Vmax, for the ith enzyme. |
We don't have a way to export this macro. | The scale factor for overall liver Vmax. This factor is the same for all enzymes and is set to a default value of 1.0. |
We don't have a way to export this macro. | The scale factor for overall liver Km. This factor is the same for all enzymes and is set to a default value of 1.0. |
The purpose of the two scale factors for Vmax and Km, respectively, is to allow optimization of these values to fit simulated metabolic rates to observations without changing the individual enzyme Vmax and Km values, which allows for comparison with experimental data. As a result, you can estimate the net effective Vmax and Km from plasma concentration-time data for several doses, which gives you two options:
You can adjust the maximum velocity and concentration sensitivity for liver metabolism while maintaining proportionally correct ratios among enzymes from their experimental data.
You can fit a net “lumped” GI metabolic rate for a single “effective” enzyme.
