Solubility and Dissolution Models
The ACAT model assumes that only drug in solution can undergo lumenal degradation, carrier-mediated influx transport, absorption into the intestinal wall via the transcellular pathway, or absorption into the portal vein via the paracellular pathway.
After drug has been absorbed, it is assumed to remain in solution in enterocytes, plasma, and all PK compartments.
Thus, both the rate at which solid drug particles dissolve and the total amount of drug that can be dissolved in any compartment at any time (the solubility) have a direct effect on the degradation, efflux, metabolism, and absorption of a drug. Some lipophilic drugs can be incorporated into bile salt micelles and achieve solubilities that are greater than in water. GastroPlus® can account for the changes in solubility due to the concentration of bile salts in each region of the small intestine and has built-in bile salt concentration data for both fed and fasted conditions for human and preclinical species. For ionizable compounds, both the total amount of material that can dissolve at any point and the rate of dissolution partially depend on the local pH. If the drug in solution transits from a compartment of high solubility to one of lower solubility, then precipitation can occur. The rate of precipitation can affect the oral plasma concentration (Cp) versus time profile.
As shown in Figure 1-1, the ACAT model differential equations define three parallel series of transit sub-compartments to accommodate unreleased drug, undissolved drug, and dissolved drug. The model can also use measurable data for predicting lumenal degradation, which is the process of degrading the dissolved drug in the dissolved sub-compartments.
Undissolved drug undergoes transit and dissolves to give dissolved drug. Dissolved drug undergoes transit, lumenal degradation, and absorption. If the concentration of the dissolved drug exceeds the local solubility within a particular compartment, then the dissolved drug can also precipitate and become undissolved.
Transit of any drug form—unreleased, undissolved, or dissolved—is allowed only to the same sub-compartment of the next compartment. For example, undissolved drug can transit only to the undissolved sub-compartment of the next compartment. Changes in form—unreleased to undissolved or dissolved, undissolved to dissolved, and dissolved to absorbed, undissolved, or degraded—are allowed to occur only within a compartment.
Solubility
The Dissolution process is the process that gets drug into solution. Solubility is one of the compound properties that must be used as an input for the Dissolution models and the Precipitation models, and therefore, is discussed first.
Generally, ionized molecules have greater aqueous solubility than their un-ionized forms; thus, organic acids are more soluble at higher pH values and organic bases are more soluble at lower pH values. This general principle is the basis for the calculation of the pH- dependence of solubility in GastroPlus®. Molecules with both acidic and basic functional groups can form zwitterions that have lower aqueous solubility than either the cationic form at low pH or the anionic form at high pH. GastroPlus® accepts compounds with multiple ionizable groups in its calculation of the total percent ionized. This allows the simulation to account for the dissolution profile of zwitterions or ampholytes.
To estimate the solubility of a drug at specific pHs, three variables are required:
Solubility factor (SF) of the drug, which is the ratio of the solubility of completely ionized drug to completely unionized drug. These values are known to vary from < 10 to over 106 for different compounds, with higher values for compounds with lower intrinsic solubilities. The rough rule for estimating the SF for a drug is:
SF = 20/(intrinsic solubility in mg/mL)
All pKas for the drug.
The solubility of the drug at any single pH.
GastroPlus® uses the following pH profiles for the human small and large intestines based on experiments performed by continuously determining the pH with time 1 2 3 .
Compartment | Human ACAT pH |
Stomach | 1.3 (fasted)/4.9 (fed) |
Duodenum | 6.0 (fasted)/ 5.4 (fed) |
Jejunum | 6.2–6.4 (fasted)/5.4–6.0 (fed) |
Ileum | 6.6–7.4 (fasted and fed) |
Caecum | 6.4 (fasted and fed) |
Colon | 6.8 (fasted and fed) |
Another important aspect that affects in vivo dissolution of a drug is the presence of bile salts in the small intestine. GastroPlus® includes an equation (Equation 1-5) to account for the difference between aqueous and in vivo solubility due to the presence of bile salts in the small intestine 4 . The calculated value is then used in the Dissolution model.
Equation 1-5: Difference between aqueous and in vivo solubility caused by the presence of bile salts
where:
Variable | Definition |
We don't have a way to export this macro. | The aqueous solubility at a given pH. |
We don't have a way to export this macro. | The aqueous solubilization capacity calculated as the ratio of moles of drug to moles of water at a concentration that is equal to aqueous solubility. |
We don't have a way to export this macro. | The drug molecular weight. |
We don't have a way to export this macro. | The bile salts concentration. |
We don't have a way to export this macro. | The solubility in the presence of bile salts at concentration [bile] and at the same pH as Cs,aq. |
We don't have a way to export this macro. | The bile salt solubilization ratio, where two options are available for estimation of this value.
|
Equation 1-6: Theoretical prediction of SR based on logP
Bile salt concentrations in individual small intestine compartments in fasted and fed state were calculated based on published values for human 6 , rat, and dog 7 . Because of the lack of information for other preclinical species, the bile salt concentration profile derived for humans is currently being used for all remaining preclinical species.
Effect of surfactants on drug solubility
Bile salts or other synthetic surfactants can be used in dissolution media to increase the solubility of the drug of interest and achieve sink conditions. The affinity to surfactants depends on the drug lipophilicity and micelle surfactant type. Drugs may be able to adsorb at the surface of the micelle, form mixed micelle with the surfactant or penetrate the micelle hydrophobic core if it exists. Other factors like pH, ionic strength for ionic micelles can also alter the partitioning of the drug to the micelles since these parameters can change the nature and intensity of intermolecular force balance for the drug in the aqueous environment and the micelle. Finally, the micelle concentration may also play a role since the size, shape and type of micellar phase may change with surfactant concentration. For very low surfactant concentrations, below the critical micelle concentration (CMC), the surfactant exists as solute in the aqueous phase. With increasing surfactant concentration, the surfactant will form aggregates, micellar, tubular, or lamellar phases. These phase transitions may affect how the drug solubilizes in the micelle. Finally, for ionic surfactants which bear a specific counterion like Na+, Br-, or Cl- in Sodium docedyl sulfate (SDS), dodecyltrimethylammoniumbromide (DTAB), or tetradecyltrimethylammonium chloride (TTAC) respectively, adding extra-counterion in the medium, typically from the buffer used to control the medium pH can affect the size and CMC of surfactants. To model the effect of surfactant on solubility and dissolution one important factor to measure is the drug affinity to the surfactant in given experimental conditions (typically those of the in vitro dissolution medium)
The affinity to surfactant: (
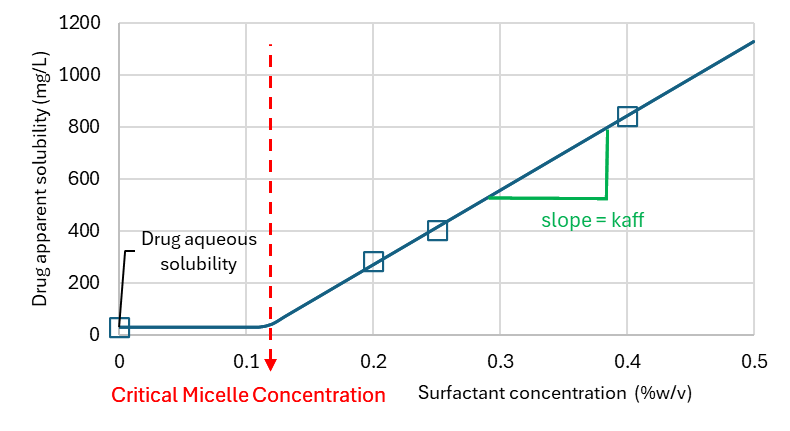
Equation 1-7: Apparent Solubility of Drug in Presence of Surfactant
Note that if
When micelles are formed is provided in mg/mL/mM surfactant or mg/mL/percent surfactant depending on the unit of surfactant concentration in the dissolution medium.
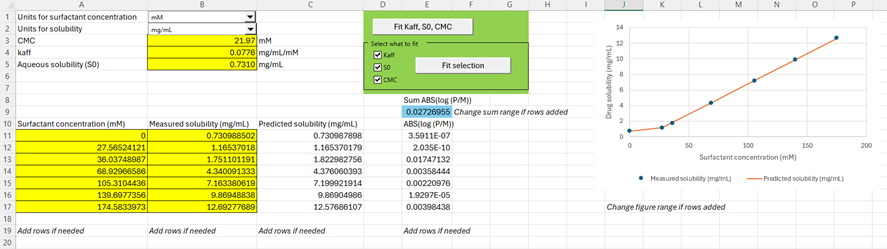
Within the Help menu in GPX™, you can access a fitting tool to calculate the parameters
Select the units for Surfactant concentration and solubility of your experiment using the drop-down menus provided. Add your solubility data as function of surfactant concentration in the range starting from A11:B11. Seven rows are provided for your data entry, and you can add more rows if needed. If you do add more rows, remember to change the sum formula of cell E9. You have two choices for fitting your parameters: either fit all parameters to the data or select the parameters you want to fit. You can manually change the
Cyclodextrin formulations for increasing aqueous solubility
Cyclodextrins are cyclic oligosaccharides. They pharmaceutical adjuvants used as complexing agents to increase the aqueous solubility of active substances that are poorly soluble in water. The molecular structure of these glucose derivatives, which approximates a truncated cone, bucket, or torus, generates a hydrophilic exterior surface and a non-polar interior cavity. Cyclodextrins can interact with appropriately sized drug molecules and bind drug molecules 1:1 into their central cavities. The binding is reversible and can be described by a simple binding constant, K_assoc, as shown in Equation 1-8.
Equation 1-8: Cyclodextrin binding constant
where:
Variable | Definition |
We don't have a way to export this macro. | The concentration of cyclodextrin-drug complex. |
We don't have a way to export this macro. | The concentration of free, dissolved drug. |
We don't have a way to export this macro. | The concentration of free cyclodextrin. |
Dissolution models
In GastroPlus®, you can calculate dissolution using one of three models:
The Johnson (default) model 8
Equation 1-9: Default model equation for dissolution rate
The Wang-Flanagan model 9 10
Equation 1-10: Wang-Flanagan model equation for dissolution rate
The Z-Factor model 11
Equation 1-11: Z-Factor model equation for dissolution rate
where:
Variable | Definition |
Note: All symbols in all three model equations represent the same properties or quantities. | |
We don't have a way to export this macro. | The amount of drug that is dissolved. |
We don't have a way to export this macro. | The effective diffusion coefficient. |
We don't have a way to export this macro. | The spherical particle radius at the current time. |
We don't have a way to export this macro. | The shape factor that accounts for non-spherical shape of the particles and is specified as the ratio of length and diameter of the particle. Note: For spherical particles, S =1. |
We don't have a way to export this macro. | The solubility at the pH of the corresponding jth intestinal compartment. |
We don't have a way to export this macro. | The total concentration of drug in the lumen of the jth intestinal compartment at time t. |
We don't have a way to export this macro. | The amount of drug that is undissolved at time = t. |
We don't have a way to export this macro. | The amount of drug that is undissolved at time =0. |
We don't have a way to export this macro. | The density of the drug. |
We don't have a way to export this macro. | The thickness of the diffusion layer. |
The z factor in Equation 1-11 represents the term shown in Equation 1-12 and is obtained by fitting the equation to in vitro dissolution data, where Dw is the diffusivity of the drug in water. The remainder of the variables are defined in the table above.
Equation 1-12: Term represented by the z factor
Five variables can be taken into consideration in a Dissolution model:
The diffusion layer thickness. See Diffusion layer thickness.
The effective diffusion coefficient. See Effective diffusion coefficient.
The particle radius. See Particle radius.
The effect of particle size on solubility. See Effect of particle size on solubility.
The aqueous solubility of the drug due to its formulation. See Cyclodextrin formulations for increasing aqueous solubility.
Diffusion layer thickness
Empirical evidence suggests that diffusion layer thickness is of the order of the particle radius for particle radii up to 30 μm. The Johnson (default) model and the Wang-Flanagan model use diffusion layer thickness that is equal to the particle radius up to an upper limit of 30 μm; however, the models also include an option to use a constant diffusion layer thickness that is independent of particle size. The diffusion layer thickness is not applicable for the Z-Factor model.
Effective diffusion coefficient
The effective diffusion coefficient (Deff) is calculated according to Equation 1-13.
Equation 1-13: Effective diffusion coefficient calculation
where:
Variable | Definition |
We don't have a way to export this macro. | The aqueous diffusion coefficient of drug monomers (free dissolved drug). |
We don't have a way to export this macro. | The fraction of free, dissolved drug calculated as the ratio of aqueous solubility and solubility in the presence of bile salts. |
We don't have a way to export this macro. | The diffusion coefficient of the bile salt-phospholipid-drug aggregate. |
Okazaki 12 has shown that the size of an aggregate, and subsequently, its diffusion coefficient, is a function of phospholipid and bile salt concentrations and is not significantly affected by saturation with the dissolved drug. The relationship between the Dagg and bile salt concentration was fitted to published data and is used within GastroPlus® to calculate Deff in each compartment. If the option to adjust the diffusion coefficient for bile salt effect is not selected, then Deff is assumed to be equal to Dmono.
Particle radius
Two particle size distribution options are available for calculating the particle radius:
Monodisperse – All particles have the same size.
Polydisperse – The formulation is a mixture of different particle sizes.
GastroPlus® has two built-in probability density functions—Log Normal and Normal—that are used for setting up initial particle size distributions. (A user-defined distribution option is also available.) These distributions are divided equally into spaced bins, and then normalized to 100% to calculate the initial mass of undissolved drug that corresponds to each bin.
Log-Normal distribution
Equation 1-14 defines the probability density function for the Legacy Log-Normal distribution.
Equation 1-14: Probability density function for the Legacy Log-Normal distribution
where:
Variable | Definition |
We don't have a way to export this macro. | The mean radius (μ) in log units as calculated from the user-specified value for the radius in microns. We don't have a way to export this macro. |
We don't have a way to export this macro. | The mean standard deviation in log units as calculated from the user-specified value of standard deviation (σ) in microns. We don't have a way to export this macro. |
Equation 1-15 is the calculation for the lower radius limit and Equation 1-16 is the calculation for the upper radius limit. These values can be edited if only a fraction of the overall distribution was used in the current formulation.
Equation 1-15: Lower radius limit, Legacy Log-Normal distribution
Equation 1-16: Upper radius limit, Legacy Log-Normal distribution
Normal distribution
Equation 1-17 defines the probability density function for the Normal distribution.
Equation 1-17: Probability density function for the Normal distribution
where:
Variable | Definition |
We don't have a way to export this macro. | The mean radius in microns. |
We don't have a way to export this macro. | The mean standard deviation in microns. |
Equation 1-18 is the calculation for the lower radius limit and Equation 1-19 is the calculation for the upper radius limit. These values can be edited if only a fraction of the overall distribution was used in the current formulation.
Equation 1-18: Lower radius limit, Normal distribution
Equation 1-19: Upper radius limit, Normal distribution
Optionally, you can decide if the dissolution process is to affect the particle radius or if the particle radius should be kept constant for the purposes of the dissolution rate calculation.
For quickly dissolving compounds, the option of keeping a constant radius can provide adequate results while increasing the simulation speed.
If the dissolution process affects the particle radius, then the particle radius is adjusted to the remaining undissolved amount as shown in Equation 1-20.
Equation 1-20: Adjusting the particle radius to the remaining undissolved amount
where:
Variable | Definition |
We don't have a way to export this macro. | The particle radius of bin k at time t. |
We don't have a way to export this macro. | The particle radius of bin k at time 0. |
We don't have a way to export this macro. | The total undissolved amount in bin k (across all compartments) at time t. |
We don't have a way to export this macro. | The total undissolved amount in bin k (across all compartments) at time 0. |
If the particle radius decreases in response to the dissolution process, then the diffusion layer thickness decreases accordingly. In all cases when particle diameters become less than 10 nm, GastroPlus® ceases to include them in the integration.
If the particles are non-spherical, then the particles in all bins are considered to be the same shape and the same shape factor is applied to all particles and is kept constant throughout the simulation.
The drug is assumed to be dissolving evenly from the entire surface of the particles and not affecting the particle shape.
Effect of particle size on solubility
The solubility for particles of a specific radius is calculated from the Kelvin equation.
Equation 1-21: Kelvin equation
where:
Variable | Definition |
We don't have a way to export this macro. | The solubility of drug particle of radius r at temperature T. |
We don't have a way to export this macro. | The solubility with no curvature at temperature T. |
We don't have a way to export this macro. | The interfacial surface tension. |
We don't have a way to export this macro. | The molar volume of drug calculated as the ratio of molecular weight and density. |
We don't have a way to export this macro. | The gas constant. |
We don't have a way to export this macro. | The drug particle radius. |
If all temperatures are specified, then the solubility with no curvature at temperature T (Cs,T) is calculated from the reference solubility as shown in Equation 1-22, with the calculation of the value of K (the reference solubility) shown in Equation 1-23.
Equation 1-22: Solubility with no curvature at temperature T
Equation 1-23: Calculation of K in Equation 1-22
where:
Variable | Definition |
We don't have a way to export this macro. | The reference solubility measured at temperature Tref. |
We don't have a way to export this macro. | The melting point of the drug. |
We don't have a way to export this macro. | The currently specified temperature. |
We don't have a way to export this macro. | The molecular weight of the drug. |
Several published studies 13 14 showed significantly higher bioavailability of poorly soluble drugs after dosing formulations consisting of nano-sized API particles when compared to formulations with particles in the micron range. The increase was much higher than expected based on a faster dissolution due solely to an increased surface area and/or to a solubility increase described by the Kelvin equation. One hypothesis is that this effect could be caused by a local '”supersaturation” when the nano-sized particles get trapped between apical microvilli. The dissolution of these trapped particles in this limited volume would result in a higher local concentration of dissolved drug, which, in turn, creates a higher apical concentration gradient and therefore increases the flux across the apical membrane.
An equation based on this “super-saturation” hypothesis has been incorporated into GastroPlus®. The equation adjusts the solubility of the compound that is used in the Dissolution model. The local supersaturation is given by the NanoFactor Effect (NFE). This effect specifies the extent by which a drug can dissolve from these nano-sized particles to concentrations that exceed the local solubility limit. The NFE for different particle sizes is calculated using an empirical equation shown in Equation 1-24.
Equation 1-24: Nanofactor Effect calculation
where:
Variable | Definition |
We don't have a way to export this macro. | The drug particle radius. |
We don't have a way to export this macro. | The NanoFactor, which is a user-defined, compound-specific parameter, with the same value being applicable across formulations with differing sizes of drug particles. |
Simulations Plus calibrated the NFE equation using the Cp versus time data for several formulations of aprepitant with particle diameters ranging from 5.5 microns to 0.12 microns. Because the equation was calibrated based on particle diameter, (2r), Simulations Plus determined the default value of NF to be 0.5.
Using the P-PSD models
If a surfactant is used in the dissolution medium, fu the fraction of free drug in the medium is given by:
Equation 1-25: Fraction of Unbound Drug in Solution
where Cu(t) is the unbound drug concentration at all times and C(t) the total drug concentration at all times. Equilibration is assumed to be instantaneous and so fu is anticipated to be constant. The affinity to surfactants kaff, is experimentally determined to apparent solubility vs surfactant concentration (Csurf) in each dissolution medium (See Effect of surfactants on drug solubility).
For the P-PSD approach, the in vitro dissolution rates are fitted according to the film theory, i.e. controlled by the drug diffusion through a stagnant film layer surrounding the dissolving particle called the unstirred water layer (UWL) 15 . The Nernst-Brunner equation, which describes the variation of amount of undissolved drug dmsolid (kg) with time for a solid particle is modified to take into consideration the binding of drug to micelles and the diffusion coefficient of micelles and pure drug out of the unstirred water layer. This equation is close to the one developed by Gamsiz et al. 16 , although the treatment of fraction drug unbound and thickness in UWL differ from the approach taken by these authors 17 .
Equation 1-26: Dissolution Rate Calculation
Where A(t) (m2) is the surface area of the particle at time t, Du and Db the diffusion coefficient of the unbound drug and micelle-bound drug respectively (m2.s-1), hu and hb the UWL thickness of the unbound drug and micelle-bound drug respectively (m), CS,u is the unbound drug solubility at the surface of the drug crystal and Cu, the unbound drug concentration in the bulk (kg.m-3). This equation sums up the flux of drug dissolving from the crystal surface as free and micelle bound drug.
The term
Equation 1-27: Dissolution Rate Expanded Equation
Where
The inclusion of hydrodynamics in the dissolution model relies on different models to calculate the thickness of the unstirred water layer (UWL). Two additional models for P-PSD are available which integrate the fluid velocity in USP2 dissolution apparatus, the P-PSD HD, and one model predicting drug and excipient sedimentation and cone formation at the bottom of the USP2 vessel, the P-PSD HDC 18 . These latter models are important to remove the potential bias coming from formulation sedimentation or to integrate the impact of fluid velocity in USP2, which would be important for large particles or large dosage forms such as eroding tablets or pellets 19 20 . The P-PSD HD and P-PSD HDC models are both available for download here ( P-PSD-HD.xlsx and P-PSD HDC-1_2_model.xlsx).
For the classical P-PSD approach, the assumption is that the thickness for the UWL of the unbound drug is assumed equivalent to the particle radius for small particles as long as the particle radius is below 30 µm and 30 µm for particles ≥ 30 μm 21 . As the dissolution proceeds, the particles shrink and this stagnant film layer will be reduced as well according to time. This approximation for the thickness of the UWL is valid for small particles. When particles will become larger or when the hydrodynamics in the model will be increased, the particles may experience different velocity profiles relative to the fluid flow which will change the thickness of the UWL. The thickness of the UWL for the micelle-bound drug is calculated based from the diffusion coefficients of the free drug and the micelle-bound drug according to the following equation 22 :
Equation 1-28: Characteristic Length Scale for Diffusion
The free drug or micelle-bound drug diffusion coefficients D (m2.s-1) are obtained from literature data or from the Stokes Einstein equation, assuming that the molecular shape is a sphere of radius rh (m):
Equation 1-29: Stokes-Einstein Equation for Diffusion Coefficient
Where k = 1.3806504 10-23 (J.K-1) is the Boltzmann constant, T is the absolute temperature in kelvin, η (Pa.s) is the dynamic viscosity of the solvent and rh (m) is the hydrodynamic radius of the diffusing solute. The hydrodynamic radius of the solute can be estimated, assuming that the molecular shape is a sphere and that the hydration of the solute is negligible, by the following equation:
Equation 1-30: Calculation of Diffusion Coefficient in Water
Where MW is the molecular weight of the drug (g.mol-1), NA = 6.02214179 1023 (mol-1) is the Avogadro number and ρS is the drug true density in (kg.m-3). The effect of temperature for dissolution is accounted for by a change in the water viscosity.
For micelles, a database of micelle size is provided in GPX™ to select baseline values for the simulation. The size of micelles is vastly different which can lead to widely different diffusion coefficients.
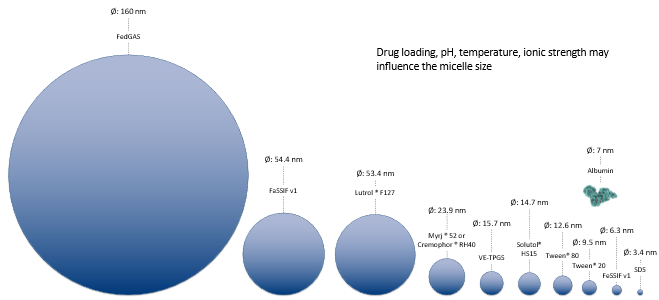
The database within GPX™ will provide starting values for micelle size. Note that since the temperature, counter-ion concentration and surfactant concentration will affect the size of micelles and also the CMC of the micelles, an additional tool is available from the help to be able to refine the size and CMC of micelles in given media. To access this tool, download the “Size and CMC calculator.xlsm” file here ( Size and CMC calculator-v1.0.xlsm).
From the welcome page, click on the button “Launch CalcSurf v1.0”.
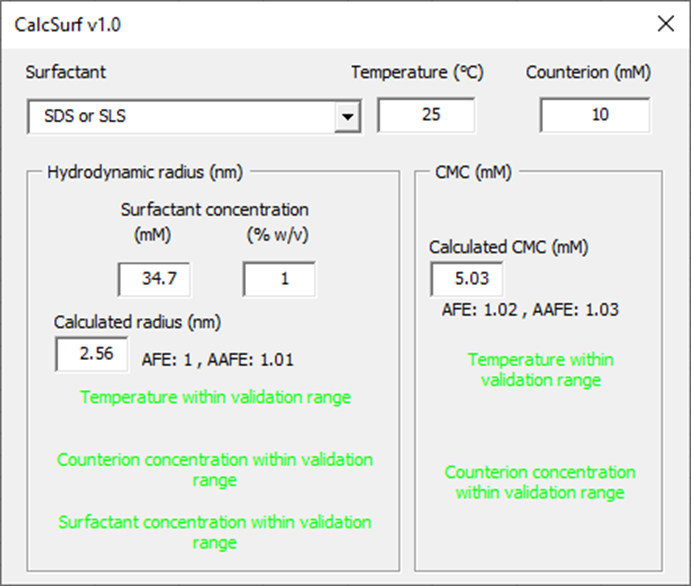
From the surfactant drop down menu, select the surfactant of interest. Indicate the temperature of the medium (°C) and counterion concentration (mM) if the surfactant is ionic. Then indicate a surfactant concentration for the calculation of micelle hydrodynamic radius. The tool will indicate whether the input parameters are within the validation range and what are the model prediction performance in terms of Average Fold Error (AFE) and Absolute Average Fold Error (AAFE). The calculated hydrodynamic radii for micelles and CMC can be used in GPX™ to inform the in vitro dissolution fitting when micelles are present.
For very small particles (in the nanosize range), the solubility is different than that of larger particles. The particle size and the interfacial tension between the solid particle and the solvent phase have to be considered with the use of the Kelvin equation:
Equation 1-31: Solubility of Drug Particles as a Function of Particle Radius
Where Cr stands for the solubility of a drug particle of radius r (m). C∞ is the solubility of the same drug with no curvature (or an infinite radius of curvature) at temperature T. Units for C∞ and Cr are the same relevant concentration units. Rg = 8.314472 J.K-1.mol-1 is the ideal gas constant, T (K) is the absolute temperature,
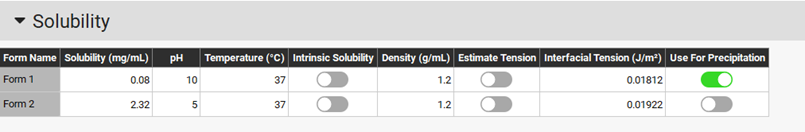
The interfacial tension for each form is taken from the solubility asset of the compounds. If surfactants are present in the dissolution medium, you may want to update the interfacial tension for the forms present in solution.
The P-PSD tool works by assigning a solid drug mass to a spherical particle size distribution of up to n=10 bins. For each bin ‘i’ in the distribution, the initial particle radius
Equation 1-32: Calculation of Number of Particles per bin
Where ρS (kg.m-3) is the material true density assuming a spherical particle size. At all times, the UWL thickness for the dissolving particles and the unbound drug is given by hu(t)= r(t) if r(t)<30 µm or hu(t)=30 µm if r(t) ≥ 30 µm. In each particle bin the mass of particles is reduced by integrating equation 9 over time.
Particles are allowed to shrink, and the surface area of particles dissolving is calculated for each time point in each bin by:
Equation 1-33: Surface Area of Dissolving Particles
The P-PSD is unique for a drug product batch and represents the impact of drug substance particle size, but also formulation or process parameters which may influence dissolution.
In case of precipitation
If several forms of the same moiety are defined as part of the formulation description, during dissolution, there may be a point where the form with the highest solubility continues to dissolve to build the bulk drug concentration, whilst the form with the lowest solubility is becoming supersaturated, i.e. the bulk drug concentration is higher than the solubility of the lowest solubility form in the medium. It is proposed for this version of the P-PSD to use the same equation for dissolution and precipitation. When the bulk concentration is higher than the solubility of one form, the difference
Equation 1-34: Rate of Change of Solid Mass During Dissolution and Precipitation
This would allow to capture the dissolution of polymorph mixtures such as those of form alpha and beta for rifaximin, for which the mixing law is not linear in terms of percent dissolved at 2 hours.
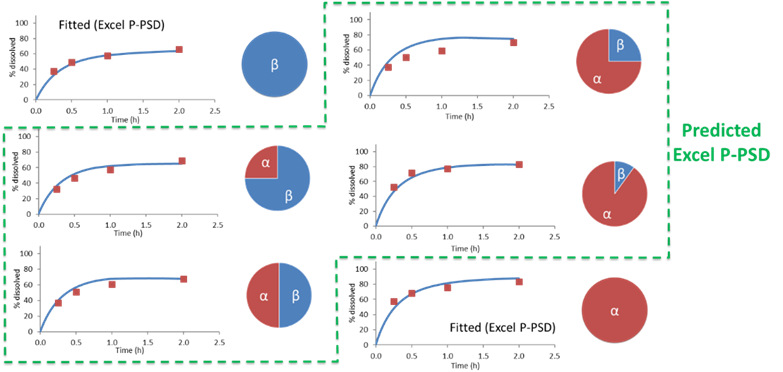
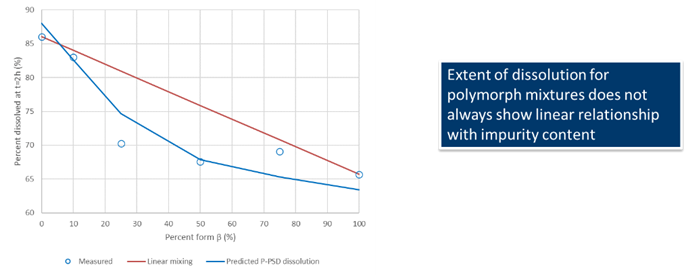
Data for Rifaximin from Dharani, S., et al. 23 , show that the mixing law for percent dissolved at 2 hours for rifaximin form alpha and beta mixture is not linear and is well predicted using the P-PSD approach.
In case of degradation
When there is drug degradation in the medium, e.g. due to pH, the solubilized drug mass will be removed as time passes. This can accelerate the apparent drug dissolution and also lead to bell shaped profiles where the drug concentration in the vessel goes through a maximum and then diminish, or goes through an apparent plateau (if the dissolution balances the degradation).
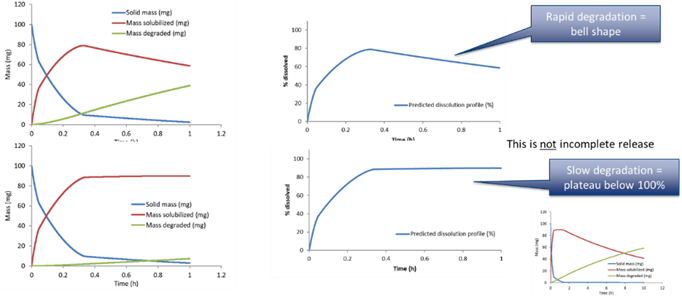
The first order drug degradation rate constant can be used to calculate the mass degraded over time using the following equation, where
Equation 1-35: First-Order Drug Degradation Rate Equation
- Dressman, J.B., Amidon, G.L., et al. (1998). “Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms.” Pharm. Res. 15(1): 11-22.
- Evans, D.F., Pye, G., et al. (1988). “Measurement of gastrointestinal pH profiles in normal ambulant human subjects.” Gut 29(8): 1035-41.
- Russell, T.L., Berardi, R.R., et al. (1993). “Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women.” Pharm. Res. 10(2): 187-96.
- Mithani, S.D., Bakatselou, V., et al. (1996). “Estimation of the increase in solubility of drugs as a function of bile salt concentration.” Pharm. Res. 13(1): 163-7.
- Mithani, S.D., Bakatselou, V., et al. (1996). “Estimation of the increase in solubility of drugs as a function of bile salt concentration.” Pharm. Res. 13(1): 163-7.
- Porter, C.J., Trevaskis, N.L., et al. (2007). “Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs.” Nat. Rev. Drug Discov. 6(3): 231-48.
- Sugano, K. (2009). “Computational oral absorption simulation for low-solubility compounds.” Chem. Biodivers. 6(11): 2014-29.
- Lu, A.T., Frisella, M.E., et al. (1993). “Dissolution modeling: factors affecting the dissolution rates of polydisperse powders.” Pharm. Res. 10(9): 1308-14.
- Wang, J. and Flanagan, D.R. (1999). “General solution for diffusion-controlled dissolution of spherical particles. 1. Theory.” J. Pharm. Sci. 88(7): 731-8.
- Wang, J. and Flanagan, D.R. (2002). “General solution for diffusion-controlled dissolution of spherical particles. 2. Evaluation of experimental data.” J. Pharm. Sci. 91(2): 534-42.
- Takano, R., Sugano, K., et al. (2006). “Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a miniscale dissolution test.” Pharm. Res. 23(6): 1144-56.
- Okazaki, A., Mano, T., et al. (2008). “Theoretical dissolution model of poly-disperse drug particles in biorelevant media.” J. Pharm. Sci. 97(5): 1843-52.
- Jinno, J., Kamada, N., et al. (2006). “Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs.” J. Control Release 111(1-2): 56-64.
- Wu, Y., Loper, A., et al. (2004). “The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human.” Int. J. Pharm. 285(1-2): 135-46.
- Okazaki, A., T. Mano, and K. Sugano, Theoretical dissolution model of poly-disperse drug particles in biorelevant media. Journal of pharmaceutical sciences, 2008. 97(5): p. 1843-52.
- Gamsiz, E.D., et al., Predicting the effect of fed-state intestinal contents on drug dissolution. Pharmaceutical research, 2010. 27(12): p. 2646-56.
- Pepin, X.J.H., et al., Bridging in vitro dissolution and in vivo exposure for acalabrutinib. Part I. Mechanistic modelling of drug product dissolution to derive a P-PSD for PBPK model input. European Journal of Pharmaceutics and Biopharmaceutics, 2019. 142: p. 421-434.
- Pepin, X., M. Goetschy, and S. Abrahmsén-Alami, Mechanistic models for USP2 dissolution apparatus, including fluid hydrodynamics and sedimentation. Journal of Pharmaceutical Sciences, 2021.
- Pepin, X.J.H., et al., Physiologically Based Biopharmaceutics Model for Selumetinib Food Effect Investigation and Capsule Dissolution Safe Space – Part I: Adults. Pharmaceutical Research, 2022.
- Scholz, A., et al., Can the USP paddle method be used to represent in-vivo hydrodynamics? J Pharm Pharmacol, 2003. 55(4): p. 443-51.
- Wang, Y., et al., Comparison and Analysis of Theoretical Models for Diffusion-Controlled Dissolution. Molecular Pharmaceutics, 2012: p. 120417094737002.
- Pohl, P., S.M. Saparov, and Y.N. Antonenko, The Size of the Unstirred Layer as a Function of the Solute Diffusion Coefficient. Biophysical Journal, 1998. 75(3): p. 1403-1409.
- Dharani, S., et al., Development and Validation of a Discriminatory Dissolution Method for Rifaximin Products. J Pharm Sci, 2019. 108(6): p. 2112-2118.
