Indirect Response PD Models
Unlike the direct response PD model, an indirect response PD model accounts for lag time in the observed PD effect as well as for hysteresis, which occurs when the same concentration of drug in plasma produces two different levels of the PD effect, depending on the time course of the plasma concentration. Five types of indirect response PD models are available in GastroPlus®. See:
Effect Compartment model
Figure 3-5: Effect Compartment model schematic (indirect link)
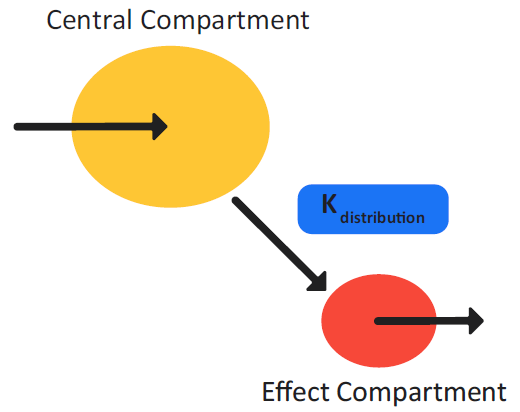
Figure 3-5 is a schematic of the Effect Compartment model. The Effect Compartment model assumes that the delay in the pharmacological action of the drug from the time that it enters the plasma compartment is due to the re-distribution of the drug to the effect compartment, which is the true site of the pharmacological action. Equation 3-7 shows the transfer rate of the drug between the two compartments.
Equation 3-7: Drug transfer rate for the Effect Compartment model
where:
Variable | Definition |
We don't have a way to export this macro. | The amount of drug in the plasma compartment. |
We don't have a way to export this macro. | The amount of drug in the effect compartment. |
We don't have a way to export this macro. | The drug transfer rate constant from the plasma compartment to the effect compartment. |
We don't have a way to export this macro. | The drug transfer rate constant from the effect compartment to the plasma compartment. |
Equation 3-8 describes the Effect Compartment model in terms of distribution of the drug.
Equation 3-8: Drug mass in the Effect Compartment model
where:
Variable | Definition |
We don't have a way to export this macro. | The rate of distribution of drug from the plasma compartment to the effect compartment, referred to as the inter-compartmental distribution rate. |
We don't have a way to export this macro. | The total or unbound concentration of drug in the plasma compartment. |
We don't have a way to export this macro. | The total or unbound concentration of drug in the effect compartment. |
The concentration of the drug in the Effect Compartment model is then linked to either the Emax model or the Sigmoid Emax model as described in Direct Response PD Models.
Class I – Class IV models
Dayneka 1 proposed a set of indirect response models that explained the lag time of the PD effect and the apparent lack of direct correlation of with Cp by means of mechanistic equations that related the Cp on the stimulation or inhibition of the generation or dissipation of the effect, where if the rate of generation and dissipation of the PD effect variable (E) in the absence of drug can be described by zero and the first-order rate constants kin and kout, respectively, then Equation 3-9 gives the rate of change of the effect variable in the absence of drug.
Equation 3-9: Rate of change of PD effect variable in the absence of drug
Under steady state conditions, kin = koutR0, where R0 is the baseline PD effect.
You can combine any of these four models with the effect compartment model to simulate indirect effect that is linked to a hypothetical effect compartment. See:
Class I and Class II indirect response models (inhibitory)
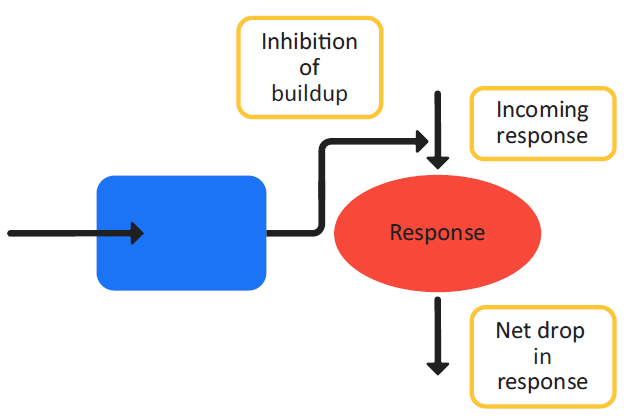
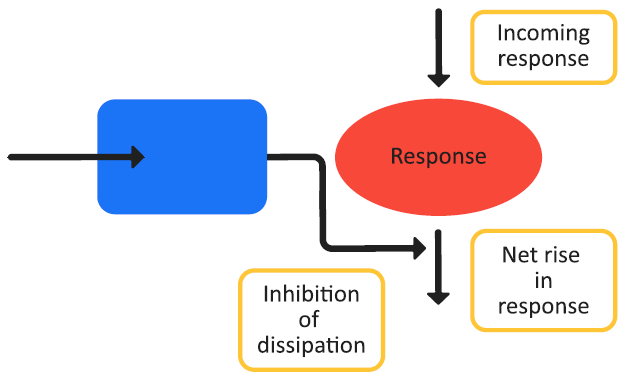
Class I and Class II indirect response models follow the standard inhibitory equation (Equation 3-10), where Class I models represent inhibitory processes that operate on the generation of the PD effect variables and Class II models represent the inhibitory processes that operate on the dissipation of the PD effect variables.
Equation 3-10: Standard inhibitory equation
Figure 3-6 is a schematic of a Class I indirect response model and Equation 3-11 shows the changes in the effect variable over time for the model.
Figure 3-6: Indirect response model, Class I
Equation 3-11: Change in PD effect variable over time, Class I indirect response model
Figure 3-7 is a schematic of a Class II indirect response model and Equation 3-12 shows the changes in the effect variable over time for the model.
Figure 3-7: Indirect response model, Class II
Equation 3-12: Change in PD effect variable over time, Class II indirect response model
For both Class I and Class II models, you must estimate the following parameters: kin, kout, Imax, and IC50.
Class III and Class IV indirect response models (stimulatory)
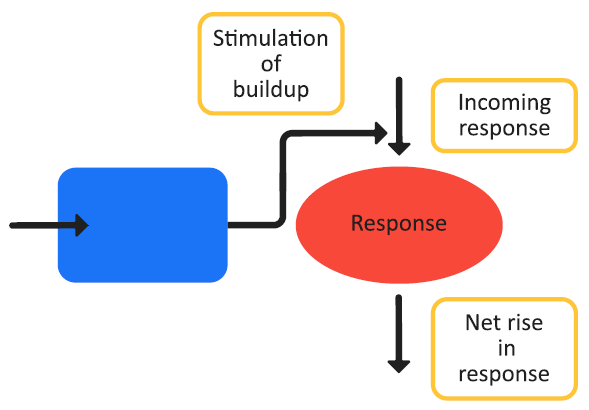
Class III and Class IV indirect response models follow the standard stimulatory equation (Equation 3-13), where Class III models represent the stimulation processes that operate on the generation of the PD effect variables and Class IV models represent the stimulation processes that operate on the dissipation of the PD effect variables.
Equation 3-13: Standard stimulatory equation
Figure 3-8 is a schematic of a Class III indirect response model and Equation 3-14 shows the changes in the effect variable over time for the model.
Figure 3-8: Indirect response model, Class III
Equation 3-14: Change in PD effect variable over time, Class III indirect response model
Figure 3-9 is a schematic of a Class IV indirect response model and Equation 3-15 shows the changes in the effect variable over time for the model.
Figure 3-9: Indirect response model, Class IV
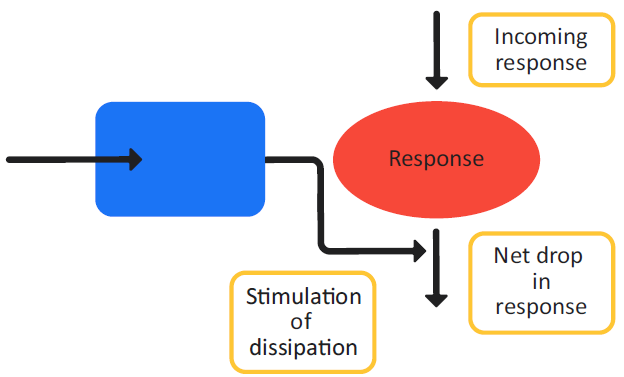
Equation 3-15: Change in PD effect variable over time, Class IV indirect response model
For both Class III and Class IV models, you must estimate the following parameters: kin, kout, Emax, and EC50.
Cell killing model: Phase non-specific
The basic pharmacodynamics model for cell proliferation and phase non-specific killing was presented by Jusko 2 and it includes the irreversible reaction between the drugs in the effect compartment and the target cells. For this model, the drug concentration in the effect compartment (Ce), which is the true site of the pharmacological reaction, can be calculated according to Equation 3-8. The rate of change in quantity of the target cells (the PD effect) is shown in Equation 3-16.
Equation 3-16: Rate of change in quantity of target cells (Cell killing model, phase non-specific)
where:
Variable | Definition |
We don't have a way to export this macro. | The cell growth rate constant, which is the difference between the natural mitotic rate and physiologic degradation. |
We don't have a way to export this macro. | The rate of the irreversible reaction. |
Bacteria killing and growth (BKG) model
Kristoffersson 3 presented the pharmacodynamics model for bacteria killing and growth. As shown in Figure 3-10, the model consists of two bacterial populations: one antibiotic susceptible population (sub-population 1, which is the main population) and one pre-existing antibiotic resistant population (sub-population 2).
Figure 3-10: Bacteria killing and growth model schematic
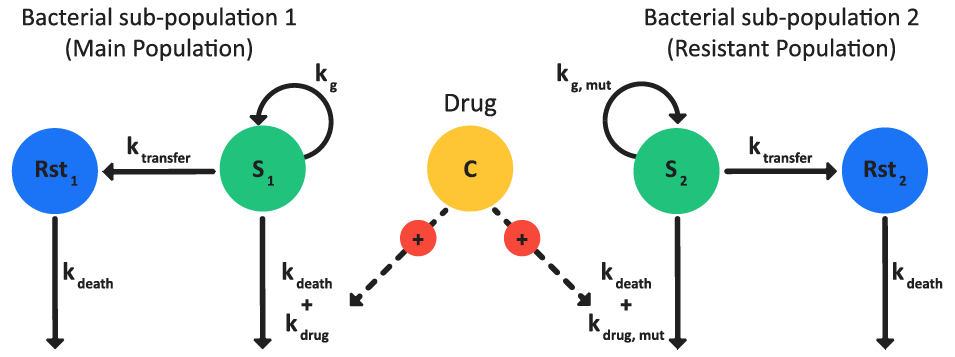
In each sub-population, the bacteria exist in two states:
Proliferating, antibiotic-susceptible (S) bacteria.
Resting (Rst), non-proliferating, antibiotic-resistant bacteria.
The initial state of the system is defined by the total number of bacteria that are in the resistant sub-population (S2 + R2) and the total number of bacteria that are in the resting state in each sub-population as shown in Equation 3-17 (an equation set).
Equation 3-17: Initial state of the system, BKG model
where:
Variable | Definition |
We don't have a way to export this macro. | The initial number of bacteria. |
We don't have a way to export this macro. | The percent of pre-existing antibiotic-resistant bacteria. |
We don't have a way to export this macro. | The percent of bacterial cells that are in the resting state. |
We don't have a way to export this macro. | Resting state of sub-population 1. |
We don't have a way to export this macro. | Resting state of sub-population 2. |
Equation 3-18 (an equation set) defines the transfer rate from the S population to the R population.
Equation 3-18: Transfer rate from S population to R population, BKG model
where:
Variable | Definition |
We don't have a way to export this macro. | The maximum number of bacteria in the stationary phase. |
We don't have a way to export this macro. | The growth rate constant for antibiotic-susceptible bacteria. |
We don't have a way to export this macro. | The rate constant for bacterial natural death. |
We don't have a way to export this macro. | The total number of bacteria across all sub-populations. |
Equation 3-19 (an equation set) describes the effect of the drug on each bacterial sub- population, where the effect is a function of the drug concentration.
Equation 3-19: Effect of the drug on each bacterial sub-population, BKG model
where kdrug,mut is the growth rate constant for pre-existing antibiotic-resistant bacteria.
Equation 3-20 (an equation set) describes how to apply these functions to study the effect of the drug using either a Power model or a Sigmoidal model, respectively.
Equation 3-20: Effect of an antibiotic drug with Power model or Sigmoidal model
where:
Variable | Definition |
We don't have a way to export this macro. | The drug concentration. |
We don't have a way to export this macro. | The Hill factor in the drug-effect relationship. |
We don't have a way to export this macro. | The maximum kill rate constant. |
We don't have a way to export this macro. | The concentration of the drug that produces 50% of Emax. |
We don't have a way to export this macro. | The shift in the concentration of the drug that is required for the drug to have the same effect on the mutant bacteria as it does on the antibiotic-susceptible bacteria, |
Precursor dependent models (Class V – VIII)
The basic indirect response models (Class I–IV) can be extended to incorporate a precursor compartment to describe the development of tolerance and rebound of the pharmacodynamics response due to the depletion or accumulation of a physiological precursor that is responsible for generating drug effects 4 5 . Figure 3-11 is a schematic of a precursor-dependent indirect model and Equation 3-21 (an equation set) shows the model calculations.
Figure 3-11: Precursor-dependent indirect response model
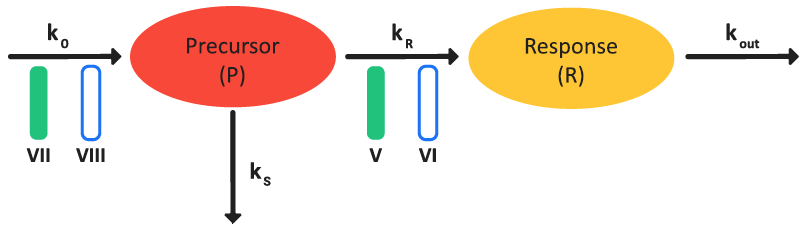
Equation 3-21: Calculations for a precursor-dependent indirect response model
where:
Variable | Definition |
We don't have a way to export this macro. | The amount of precursor. |
We don't have a way to export this macro. | The zero order rate constant for precursor production. |
We don't have a way to export this macro. | The first order rate constant for response production. |
We don't have a way to export this macro. | The first order rate constant for loss of precursor. |
We don't have a way to export this macro. | The first order rate constant for loss of response. |
We don't have a way to export this macro. | The inhibition (Class VII) or stimulation (Class VIII) of precursor production. |
We don't have a way to export this macro. | The inhibition (Class V) or stimulation (Class VI) of response production. |
To incorporate a circadian rhythm in the baseline response, the kR can be characterized as a cosine function as shown in Equation 3-22.
Equation 3-22: Incorporating a circadian rhythm in the baseline response, precursor-dependent indirect response model
where:
Variable | Definition |
We don't have a way to export this macro. | The mean production of response rate. |
We don't have a way to export this macro. | The amplitude of rate. |
We don't have a way to export this macro. | The time of occurrence of the peak production rate. |
- Dayneka, N.L., Garg, V., et al. (1993). “Comparison of four basic models of indirect pharmacodynamic responses.” J. Pharmacokinet. Biopharm. 21(4): 457-78.
- Jusko, W.J. (1971). “Pharmacodynamics of chemotherapeutic effects: dose-time- response relationships for phase-nonspecific agents.” J. Pharm. Sci. 60(6): 892-5.
- Kristoffersson, A.N., David-Pierson, P., et al. (2016). “Simulation-Based Evaluation of PK/PD Indices for Meropenem Across Patient Groups and Experimental Designs.” Pharm. Res. 33(5): 1115-1125.
- Felmlee, M.A., Morris, M.E., et al. (2012). “Mechanism-based pharmacodynamic modeling.” Methods Mol. Biol. 929: 583-600.
- Sharma, A., Ebling, W.F., et al. (1998). “Precursor-dependent indirect pharmacodynamic response model for tolerance and rebound phenomena.” J. Pharm. Sci. 87(12): 1577-84.
