Tissue Types in a PBPK Model
Fourteen default tissue types are available in GastroPlus® for physiological modeling, including liver, kidney, and lung. See:
Liver tissue including enterohepatic circulation
For details about EHC implementation with compartmental PK models, see Enterohepatic Circulation (EHC) with compartmental PK models.
In GastroPlus®, EHC can be incorporated into the pharmacokinetics of any drug. With PBPK, the biliary clearance, CLbile, can be modeled in one of two ways:
By specifying it as a fraction of general liver clearance, which is similar to biliary clearance in Compartmental PK. See Equation 2-8, which defines the biliary clearance as a fraction of total liver clearance and Compartmental Pharmacokinetics.
By specifying the passive or carrier-mediated transport of drug from hepatocytes to bile through the apical membrane. While passive and carrier-mediated transport of drug across the basolateral membrane of a tissue can be specified only for permeability-limited tissues, such transport can be specified in either a permeability- limited or perfusion-limited liver tissue model. See Equation 2-9, which defines the biliary clearance as the linear transport (passive diffusion) of drug from hepatocytes to bile.
Equation 2-8: Biliary clearance as a fraction of total liver clearance
where:
Variable | Definition |
We don't have a way to export this macro. | The biliary clearance. |
We don't have a way to export this macro. | The total liver clearance. |
We don't have a way to export this macro. | The biliary clearance fraction, which is the fraction of total liver clearance that is the result of the extraction of the drug to bile. |
Equation 2-9: Biliary clearance as the linear transport of drug from hepatocytes to bile
where PStcapical is the permeability-surface area product for the apical membrane. You can specify this value directly; otherwise it is calculated from the permeability of the drug and the apical surface area in the liver.
Equation 2-10 defines the biliary clearance as non-linear transport (active transport or carrier-mediated transport such as by the MDR1 or the MRP family of efflux transporters) of drug from hepatocytes to bile for a perfusion-limited tissue model. Equation 2-11 defines this clearance for a permeability-limited tissue model.
Equation 2-10: Biliary clearance as the non-linear transport of drug from hepatocytes to bile (perfusion-limited tissue model)
Equation 2-11: Biliary clearance as the non-linear transport of drug from hepatocytes to bile (permeability-limited tissue)
where:
Variable | Definition |
We don't have a way to export this macro. | The biliary clearance. |
We don't have a way to export this macro. | The total number of apical transporters that are involved in biliary clearance. |
We don't have a way to export this macro. | The maximum velocity of the ith transporter/enzyme. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith transporter/enzyme. |
We don't have a way to export this macro. | The concentration of unbound drug in the tissue. |
We don't have a way to export this macro. | The concentration of unbound drug in the intracellular space in the tissue compartment. |
GastroPlus® does not distinguish the importance of individual forms of biliary clearance and uses all forms that you specify. Equation 2-12, which is the sum of (Equation 2-8 + Equation 2-9 + Equation 2-10) shows how the total amount of drug that is cleared by biliary excretion for a perfusion-limited tissue model is calculated in GastroPlus®. Equation 2-13 shows how this value is calculated for a permeability-limited tissue model in GastroPlus®.
Equation 2-12: Total amount of drug cleared by excretion (perfusion-limited tissue model)
Equation 2-13: Total amount of drug cleared by excretion (permeability-limited tissue model)
where:
Variable | Definition |
We don't have a way to export this macro. | The total mass of the drug that is cleared by biliary excretion. |
We don't have a way to export this macro. | The expression of the ith transporter on the liver apical (canalicular) membrane. |
We don't have a way to export this macro. | The maximum velocity of the ith transporter/enzyme. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith transporter/enzyme. |
We don't have a way to export this macro. | The concentration of unbound drug in the tissue. |
We don't have a way to export this macro. | The concentration of unbound drug in the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The (permeability*surface area) product for the liver apical membrane. |
We don't have a way to export this macro. | The total mass of the drug that is cleared by the liver. |
We don't have a way to export this macro. | The biliary clearance fraction, which is the fraction of total liver clearance that is the result of the extraction of the drug to bile. |
For species with a gallbladder, the amount of drug that is cleared through bile is split into two parts:
One part goes to the gallbladder where it is stored during fasted state. This part is calculated as the amount of drug cleared through bile by a gallbladder fraction (fgb).
The remaining part continuously recirculates to the duodenum, where it is available for re-absorption.
When transitioning to a fed state, the fraction of drug that is stored in the gallbladder is emptied into the duodenum over the specified time period, which is referred to as the Gallbladder Emptying Time (GET).
Kidney tissue models in GastroPlus®
For many drugs, the kidneys are a major elimination route. Renal clearance is affected by a combination of four biological functions: filtration, re-absorption, secretion, and metabolism. Equation 2-14 shows the calculation that is used to generally describe kidney clearance.
Equation 2-14: General calculation for kidney clearance
where:
Variable | Definition |
We don't have a way to export this macro. | Kidney filtration clearance. |
We don't have a way to export this macro. | Clearance due to active and passive secretion. |
We don't have a way to export this macro. | Clearance due to active and passive reabsorption. |
We don't have a way to export this macro. | Clearance due to metabolism. |
When a drug is distributed to the kidneys, a fraction of the drug can be diverted to the kidney tubules. The remainder of the drug that is not filtered can partition in to the kidney cells, where it can be metabolized or secreted into the kidney tubules. In the kidney tubules, the drug can be reabsorbed into the kidney cells or excreted in the urine. To account for this filtration, re-absorption, and secretion, the GastroPlus® PBPK kidney tissue model contains an additional compartment that represents the kidney tubule. Figure 2-3 is a schematic of the perfusion-limited kidney tissue model in GastroPlus®. Figure 2-4 is a schematic of the permeability-limited kidney tissue model in GastroPlus®.
Figure 2-3: Schematics for a perfusion-limited kidney tissue model in GastroPlus®
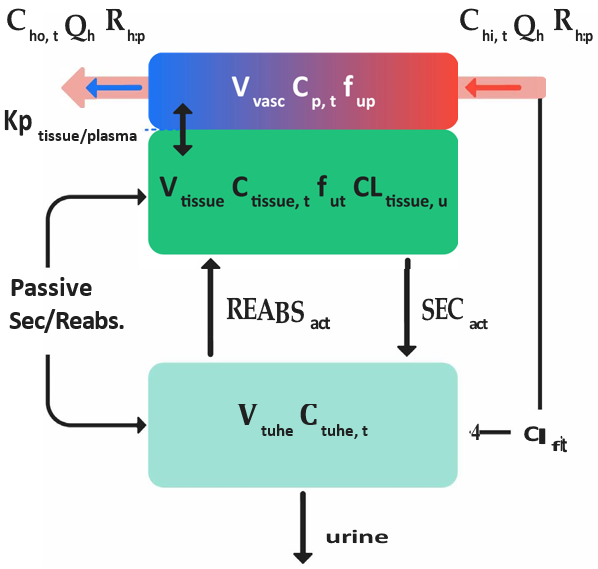
where:
Variable | Definition |
We don't have a way to export this macro. | The local tissue blood flow rate. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in the kidney tissue. |
We don't have a way to export this macro. | The blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The volume of the plasma compartment in the tissue and the total concentration of the drug in the plasma compartment in the tissue. |
We don't have a way to export this macro. | The volume of the tissue compartment and the total concentration of the drug in the tissue compartment, respectively. |
We don't have a way to export this macro. | The intrinsic clearance rate for the unbound drug from the kidney tissue. |
We don't have a way to export this macro. | The fraction of drug unbound in the plasma and the kidney tissue, respectively. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The kidney filtration clearance rate. |
We don't have a way to export this macro. | The total volume of the kidney tubules and the total concentration of drug in the kidney tubules. |
We don't have a way to export this macro. | The urine flow rate through the kidney. |
We don't have a way to export this macro. | Active secretion and re-absorption of the drug, respectively. |
We don't have a way to export this macro. | Net passive diffusion between the kidney tissue and the kidney tubules. |
Figure 2-4: Schematics for a permeability-limited kidney tissue model in GastroPlus®
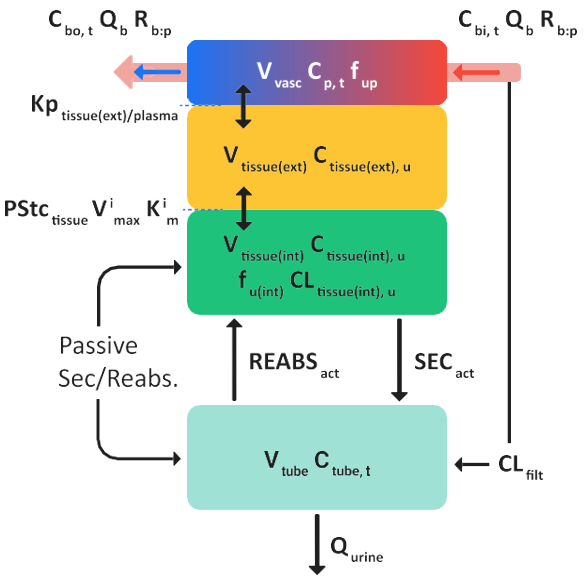
where:
Variable | Definition |
We don't have a way to export this macro. | The local tissue blood flow rate. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in the tissue. |
We don't have a way to export this macro. | The blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The volume of the plasma compartment in the tissue and the total concentration of the drug in the plasma compartment in the tissue, respectively. |
We don't have a way to export this macro. | The fraction of drug unbound in the plasma and the intracellular space in the tissue compartment, respectively. |
We don't have a way to export this macro. | The volume of the extracellular space in the tissue compartment and the concentration of unbound drug in the extracellular space in the tissue compartment. |
We don't have a way to export this macro. | The extracellular fluid to tissue partition coefficient. |
We don't have a way to export this macro. | The (permeability*surface area) product for the kidney tissue. |
We don't have a way to export this macro. | The volume of the intracellular space in the tissue compartment and the concentration of unbound drug in the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The intrinsic clearance rate of the unbound drug in the intracellular space in the kidney tissue. |
We don't have a way to export this macro. | The kidney filtration clearance rate. |
We don't have a way to export this macro. | The total volume of the kidney tubules and the total concentration of drug in the kidney tubules. |
We don't have a way to export this macro. | The urine flow rate through the kidney. |
We don't have a way to export this macro. | The maximum velocity of the ith transporter/enzyme. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith transporter/ enzyme. |
We don't have a way to export this macro. | Active secretion and re-absorption of the drug, respectively. |
We don't have a way to export this macro. | Net passive diffusion between the kidney tissue and the kidney tubules. |
Estimation of kidney filtration values (CLfilt)
The GastroPlus® PBPK Kidney model provides four methods for estimating kidney filtration:
fup * Glomerular Filtration Rate (GFR), which is the recommend method.
The Glomerular Filtration Rate (GFR).
(fraction * kidney blood flow), which is specified as a fraction of the kidney blood flow.
A user-specified renal clearance value.
The passive diffusion that occurs between kidney tissue and the kidney tubules can be specified through a permeability*surface area value, PStcapical, for the apical membrane or, if the apical surface area for the kidney is > 0, then GastroPlus® calculates the value from the permeability of the drug and apical surface area.
Active transport processes and metabolic clearance rates in the kidney tissue are calculated the same way as for general perfusion-limited and permeability-limited tissues. See Tissue Models for PBPK.
Lung tissue models in GastroPlus®
The GastroPlus® PBPK lung tissue model includes additional mechanisms for drug partitioning into the epithelial lining fluid (ELF). If an ELF/unbound plasma partition coefficient defines the partitioning, then you can model the partitioning as fast. Otherwise, depending on the tissue model type that you select, perfusion-limited or permeability-limited, you can define the partitioning as a combination of slow diffusion and carrier-mediated transport between the lung tissue and ELF1. See:
Perfusion-limited lung tissue models
If the permeability of a drug is high, then the blood flow rate (perfusion rate) through the tissue limits the amount of drug that partitions into the tissue and a tissue/plasma partition coefficient, Kptissue/plasma, is used to calculate the amount of drug that is in the tissue. The amount of drug that is in the ELF is then calculated based on one of two assumptions, which results in two different perfusion-limited lung tissue models in GastroPlus®:
An assumption of instant partitioning based on the ELF/unbound plasma partition coefficient, KpELF/plasma,u. See Perfusion-limited lung tissue model.
An assumption of slow diffusion and/or carrier-mediated drug transport across the ELF/tissue membrane. See Hybrid perfusion-limited lung tissue model.
Perfusion-limited lung tissue model
Figure 2-5: Schematic for a perfusion-limited lung tissue model in GastroPlus®
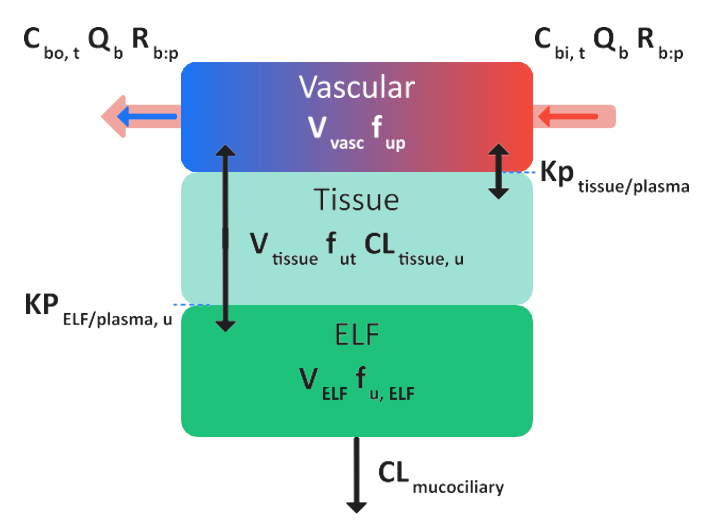
The default physiological parameters for ELF (volume, mucociliary clearance rate and pH) are estimated from the values in the PCAT module. The PCAT module includes physiologies for human, rat, mouse, and dog. The parameters for the remaining animal species are based on the existing animal physiologies in the PCAT module. For monkey and mini-pig, the ELF volume (VELF) is estimated from the total lung volume using the same ratio of ELF and lung volumes as in adult human. The mucociliary clearance rate (CLmucociliary) is approximated with the human value. For rabbit, VELF is estimated from the total lung volume using an average ratio of ELF and lung volumes across rat and mouse and CLmucociliary is approximated as an average across rat and mouse. For cat, VELF is estimated from the total lung volume using the same ratio of ELF and lung volumes as in dog and CLmucociliary is approximated with the dog value. The default ELF pH value is set to 6.69 in all species
The values for fu,ELF and KpELF/plasma,u in the table below are user-defined values. If you have a license to the ADMET Predictor module, then initial estimates for these values are available. See Quantitative Structure-Activity Relationship Predictions of ELF/ unbound plasma partition coefficients and fraction unbound in ELF.
where:
Variable | Definition |
We don't have a way to export this macro. | The local tissue blood flow rate. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in tissue. |
We don't have a way to export this macro. | The blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The volume of the plasma compartment in the tissue. |
We don't have a way to export this macro. | The volume of the tissue compartment. |
We don't have a way to export this macro. | The fraction of drug unbound in the plasma and the tissue, respectively. |
We don't have a way to export this macro. | The intrinsic clearance rate for the unbound drug from the lung tissue. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The volume of ELF. |
We don't have a way to export this macro. | The fraction of unbound drug in ELF. |
We don't have a way to export this macro. | The ELF/unbound plasma partition coefficient. |
We don't have a way to export this macro. | The mucociliary clearance rate, which is the removal of drug with the flow of ELF. |
Equation 2-15: Perfusion-limited GastroPlus® PBPK lung tissue model calculations
where:
Variable | Definition |
We don't have a way to export this macro. | The total concentration of drug in the lung tissue, at time t. |
We don't have a way to export this macro. | The total concentration of drug in lung tissue ELF, at time t. |
We don't have a way to export this macro. | The saturable clearance rate of drug calculated using Michaelis-Menten kinetics. See Equation 2-3. |
Hybrid perfusion-limited lung tissue model
Figure 2-6: Schematic for a hybrid perfusion-limited lung tissue model in GastroPlus®
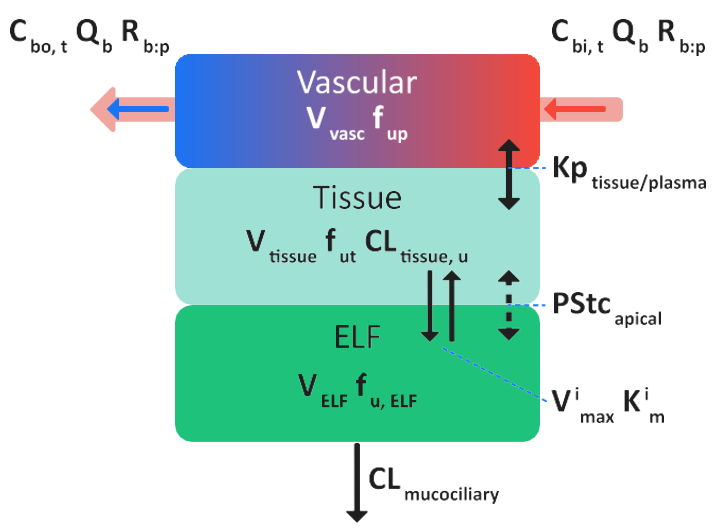
where:
Variable | Definition |
We don't have a way to export this macro. | The local tissue blood flow rate. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in tissue. |
We don't have a way to export this macro. | The blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The volume of the plasma compartment in the tissue. |
We don't have a way to export this macro. | The volume of the tissue compartment. |
We don't have a way to export this macro. | The fraction of drug unbound in the plasma and the tissue, respectively. |
We don't have a way to export this macro. | The intrinsic clearance rate for the unbound drug from the lung tissue. |
We don't have a way to export this macro. | The tissue/plasma partition coefficient. |
We don't have a way to export this macro. | The volume of the ELF. |
We don't have a way to export this macro. | The fraction of unbound drug in ELF. |
We don't have a way to export this macro. | The mucociliary clearance rate, which is the removal of drug with the flow of ELF. |
We don't have a way to export this macro. | (Apical permeability*surface area) product for passive diffusion between lung tissue and ELF. |
We don't have a way to export this macro. | The maximum velocity of the ith influx/efflux transporter. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith influx/efflux transporter. |
Equation 2-16: Hybrid perfusion-limited GastroPlus® PBPK lung tissue model calculations
where:
Variable | Definition |
We don't have a way to export this macro. | The total concentration of drug in the lung tissue, at time t. |
We don't have a way to export this macro. | The total concentration of drug in ELF, at time t. |
We don't have a way to export this macro. | The process of drug exchange between tissue and ELF via a combination of passive processes and transporter-mediated processes. |
We don't have a way to export this macro. We don't have a way to export this macro. | Represent saturable carrier-mediated transport of drug between the lung tissue and ELF calculated using Michaelis-Menten kinetics. |
We don't have a way to export this macro. | The neutral, unbound concentration of drug in the tissue. |
We don't have a way to export this macro. | The neutral unbound concentration of drug in ELF. |
Note: All other variables have been defined previously for Equation 2-15 and Figure 2-6. | |
Permeability-limited lung tissue model
If the permeability of a drug is low, then the amount of drug that partitions from plasma into the tissue is limited by one or both of the following factors:
By the permeability and the surface area that is available for permeation.
By saturable transport mechanisms.
The drug transfer between the intracellular space in the tissue compartment and the ELF is also assumed to be permeability-limited and is characterized by one or both of the following variables:
An apical PStc (PStcapical).
Carrier-mediated drug transport via influx and efflux transporters.
Figure 2-7: Schematic for a permeability-limited lung tissue model in GastroPlus®
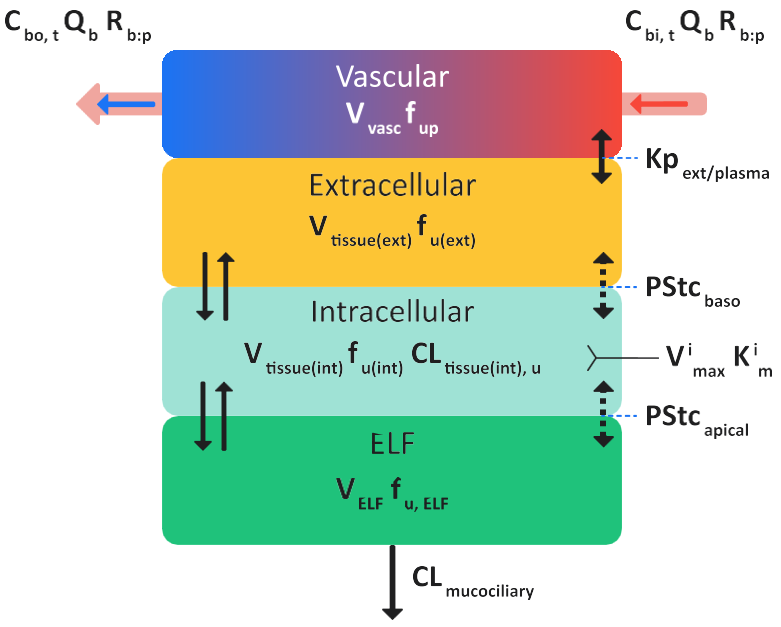
where:
Variable | Definition |
We don't have a way to export this macro. | The local tissue blood flow rate. |
We don't have a way to export this macro. | The total concentration of drug in arterial (in) blood and venous (out) blood in tissue. |
We don't have a way to export this macro. | The blood/plasma drug concentration ratio. |
We don't have a way to export this macro. | The volume of the plasma compartment in the tissue. |
We don't have a way to export this macro. | The fraction of unbound drug in the plasma. |
We don't have a way to export this macro. | The extracellular fluid to plasma partition coefficient. |
We don't have a way to export this macro. | The volume of the extracellular space in the tissue compartment |
We don't have a way to export this macro. | The fraction of unbound drug in the extracellular space in the tissue compartment |
We don't have a way to export this macro. | The volume of the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The fraction of unbound drug in the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The intrinsic clearance rate of the unbound drug in the intracellular space in the lung tissue. |
We don't have a way to export this macro. | The volume of ELF. |
We don't have a way to export this macro. | The fraction of unbound drug in ELF. |
We don't have a way to export this macro. | The (permeability*surface area) product between extracellular water and intracellular water. |
We don't have a way to export this macro. | The (permeability*surface area) product between intracellular water and ELF. |
We don't have a way to export this macro. | The mucociliary clearance rate, which is the removal of drug with the flow of ELF. |
We don't have a way to export this macro. | The maximum velocity of the ith influx/efflux transporter. |
We don't have a way to export this macro. | The Michaelis-Menten constant of the ith influx/efflux transporter. |
Similar to the perfusion-limited lung tissue model, in the permeability-limited lung tissue model, blood flows into the lung tissue with a user-specified blood to plasma concentration ratio (Rbp) and a user-specified fraction unbound in plasma (fup). The unbound fraction in plasma is in equilibrium with the fraction unbound in extracellular water (fu(ext)). See Equation 2-17 (an equation set).
All equations in Equation 2-17 account for drug exchange between the intracellular space in the tissue compartment and lysosomal compartments.
Equation 2-17: Permeability-limited GastroPlus® PBPK lung tissue model calculations
where:
Variable | Definition |
We don't have a way to export this macro. | The saturable clearance rate of drug calculated using Michaelis-Menten kinetics. |
We don't have a way to export this macro. | The total concentration of drug in the lysosomal compartments, at time t. |
We don't have a way to export this macro. | The concentration of neutral, unbound drug in the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The concentration of neutral, unbound drug in the lysosomal compartment. |
We don't have a way to export this macro. | The total concentration of drug in ELF, at time t. |
We don't have a way to export this macro. | The exchange of drug between tissue and ELF via a combination of passive processes and transporter-mediated processes. |
We don't have a way to export this macro. | The concentration of unbound drug in ELF. |
We don't have a way to export this macro. | The basolateral (permeability*surface area) product for passive diffusion between extracellular water and the intracellular space in the tissue compartment. |
We don't have a way to export this macro. | The apical (permeability*surface area) product for passive diffusion between the intracellular space in the tissue compartment and ELF. |
We don't have a way to export this macro. We don't have a way to export this macro. | The saturable, carrier-mediated transport of drug (into tissue and out of tissue, respectively) using Michaelis-Menten kinetics. |
We don't have a way to export this macro. We don't have a way to export this macro. | The saturable, carrier-mediated transport of drug between the lung tissue and ELF calculated using Michaelis-Menten kinetics. |
Quantitative Structure-Activity Relationship Predictions of ELF/ unbound plasma partition coefficients and fraction unbound in ELF
A Quantitative Structure-Activity Relationship (QSAR) model was built to predict the ELF/unbound plasma partition coefficient, KpELF/plasma,u, from the 2D structure based on literature data 1 2 . The ADMET Modeler module of ADMET Predictor was used to generate an artificial neural network ensemble (ANNE) models of the common log- transformed ELF to plasma concentration (EPR) values.The octanol to water partition coefficient model (S+logP) in ADMET Predictor was added to the descriptor pool. Models of different architecture (various number of neurons and descriptors) were built using different methods such as Kohonen maps and stratified sampling, to generate the test set. Different sensitivity analysis methods such as input gradient and genetic algorithm were used to select the descriptors. The best model was selected based on an analysis of three metrics: the root mean square error (RMSE), Spearman's rank correlation coefficient, and the coefficient of determination.
The regression model was trained on 56 compounds, with 12 compounds used as a test set to evaluate the model's predictions on compounds that fell outside the training set. The regression model was developed in log space, and the RMSE for the training and test sets were 0.335 and 0.336, respectively. As a result, the predictions typically fell within approximately two-fold of the experimental value. The square of Pearson's correlation coefficient (R2) was 0.832 for the training set and 0.752 for the test sets. Figure 2-8 shows the plot of observed versus predicted log (EPR) (log KpELF/plasma,u).
Figure 2-8: Observed versus predicted log (EPR) (log KpELF/plasma,u)
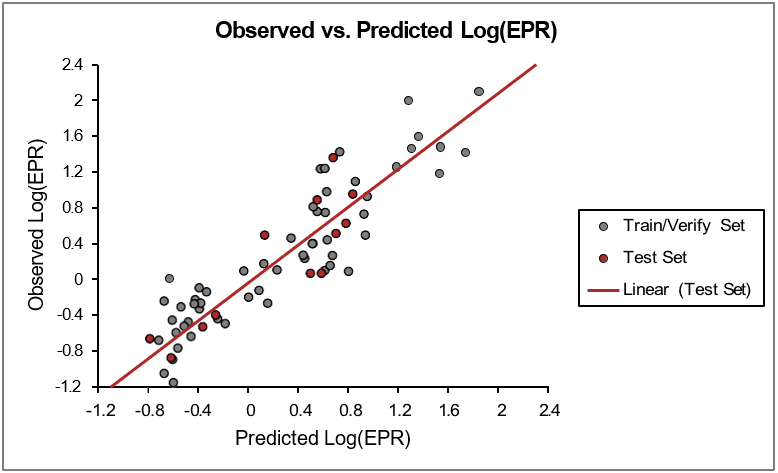
To estimate the default value for the fraction of drug unbound in ELF from the value for KpELF/plasma,u and the ionization of the drug, the assumption is made that the neutral, unbound concentration of the drug in plasma is in equilibrium with the neutral, unbound concentration in ELF. This is the same assumption that is made in the default method for the estimation of fu,tissue in a perfusion-limited tissue.
Equation 2-18: Default value for the fraction of drug unbound in ELF calculation
where:
Variable | Definition |
We don't have a way to export this macro. | The fraction of neutral, unbound drug at the pH of plasma. |
We don't have a way to export this macro. | The fraction of neutral, unbound drug at the pH of ELF. |
- Aulin, L.B.S., Valitalo, P.A., et al. (2018). “Validation of a Model Predicting Anti-infective Lung Penetration in the Epithelial Lining Fluid of Humans.” Pharm. Res. 35(2): 26.
- Välitalo, P.A., Griffioen, K., et al. (2016). “Structure-Based Prediction of Anti-infective Drug Concentrations in the Human Lung Epithelial Lining Fluid.” Pharm. Res. 33(4): 856-67.
