Dosing View
The Dosing View has two panels. All the inputs and options on these two panels (and where applicable, their sub-panels) are described below. See:
Formulations Panel
The Formulations panel is where details of the dose formulation, including route of administration, are entered.
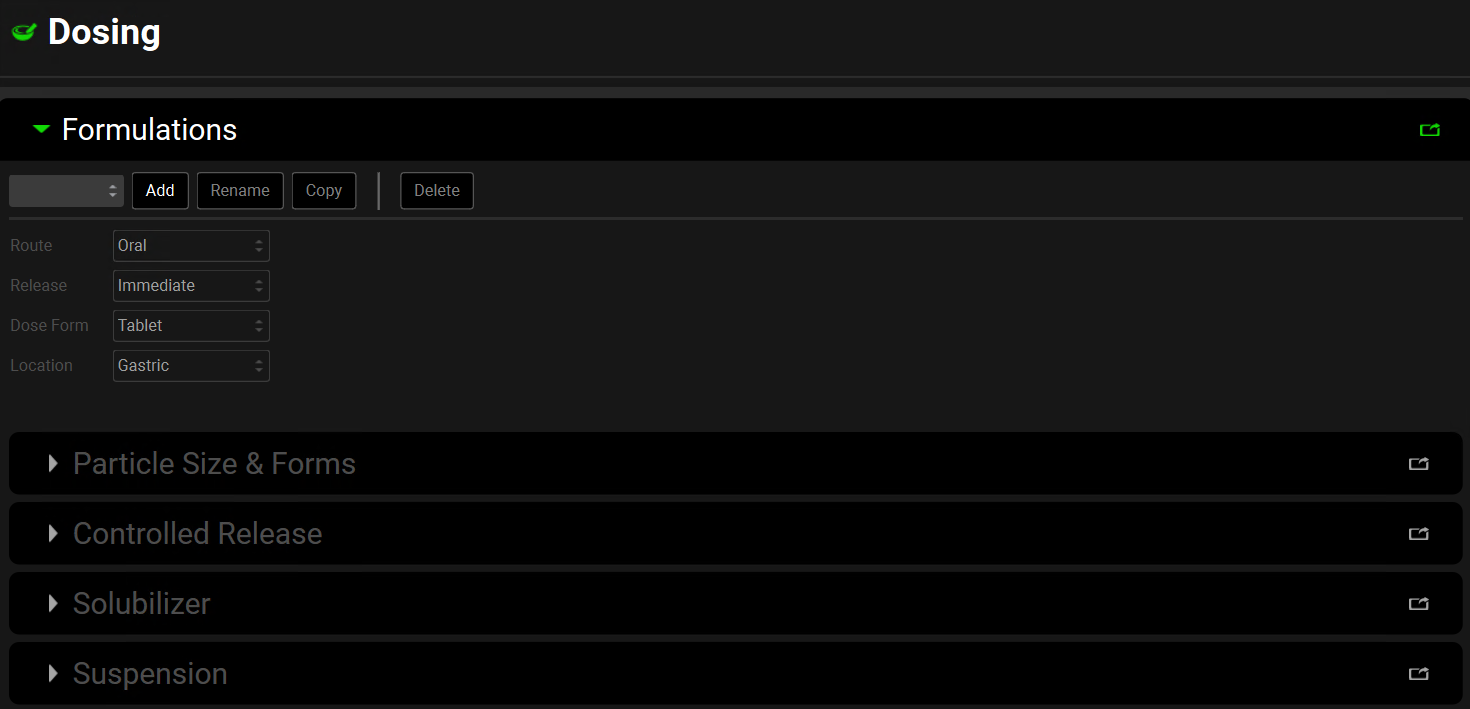
Dosing view, Formulations panel
Input/Option | Description |
Formulation Name | A drop-down. Selects the formulation.
|
Route | A drop-down. Selects the route of administration of the current formulation – Oral; IntraVenous (IV); Intramuscular. Subcutaneous, SimplifiedDose. The selection here will determine what options are available below. |
Release | A drop-down. Selects the release profile of the formulation, which controls when the drug is able to start to dissolve. Oral formulations may be Immediate, Delayed or Controlled release. Intramuscular and Subcutaneous may be Immediate or Controlled release. IV and Simplified formulations are only able to be Immediate release. |
Dose Form | A drop-down. Selects the method/form of administration. IV formulations may be:
Oral Immediate Release formulations may be:
Oral Delayed Release formulations may be:
Oral Controlled Release formulations may be:
For Intramuscular and Subcutaneous, the Dose Form drop-down is only available when Release is set to Immediate. It may be:
In all cases, release mechanism—solid particle (undissolved) or in solution (dissolved)—is determined by controlled release (see Controlled Release Sub-panel) settings. |
Location | A drop-down. Selects the location for the administration of the formulation – defaults to Venous for IV formulations and Gastric for oral formulations. Intramuscular route may be:
Subcutaneous route may be:
|
Oral, Intramuscular and Subcutaneous Formulations have a number of additional options that activate in the subsequent sub-panels when these Routes are selected.
Particle Size & Forms Sub-panel
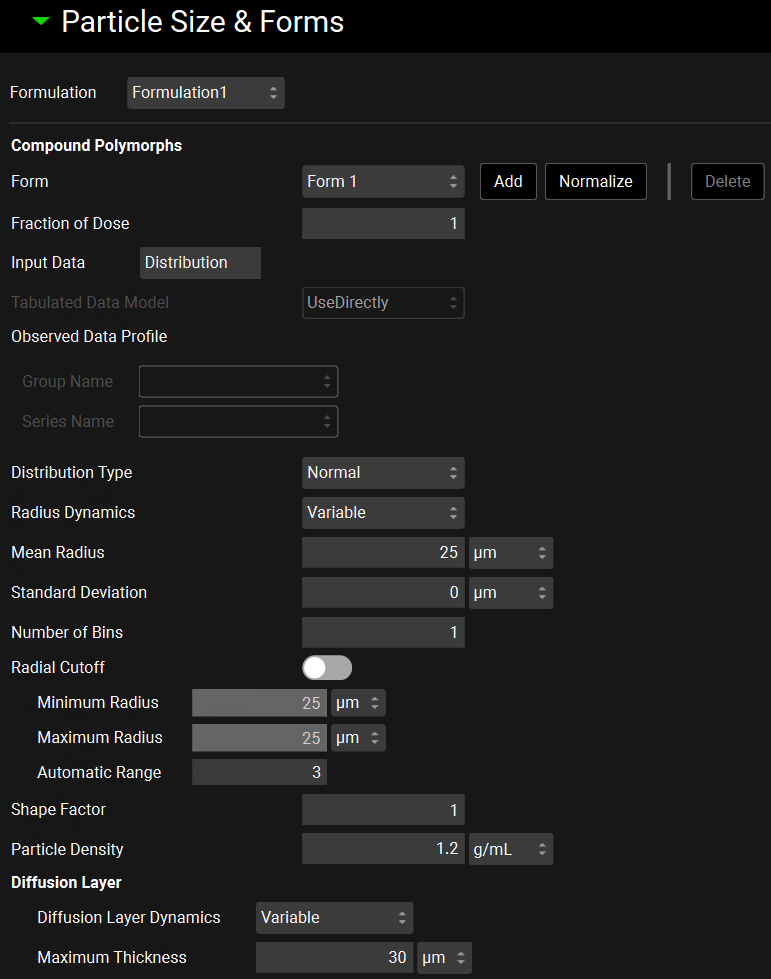
Dosing view, Formulations panel, Particle Size & Forms Sub-panel
Input/Option | Description |
Form | A drop-down list that selects the form to which the below settings will be applied. This list is not automatically populated from the forms added to the Solubility table, in the Compounds view. |
Fraction of Dose | The fraction of the dose comprised of the selected form. The fractions for all forms must add up to 1. There is a Normalize button to normalize the forms. |
Input Data | Determines the source of the particle size. Distribution uses the distribution type, mean, and standard deviation input below. Tabulated uses data entered in Observed Data view. |
Tabulated Data Model | A dropdown list that is active when Tabulated is selected as Input Data. Selects the model to which below settings are applied.
|
Observed Data Profile | Activated when Tabulated Input Data is selected. Determines which observed particle size distribution data to use.
|
Distribution Type | Activated when Distribution Input Data is selected. A drop-down list that selects which built-in Distribution Type to use to calculate the radius and mass fraction of each bin, options are Normal, Log Normal, or Legacy Log Normal (which matches the log normal distribution used in earlier versions of GastroPlus®). |
Radius Dynamics | A drop-down list that selects whether variable radius dynamics will be used – Variable - or whether a constant particle radius will be kept in each bin during the simulation - Constant. |
Mean Radius Median Radius | Activated when Distribution Input Data is selected. For Normal distribution the Mean Radius of the particles in the Formulation in µm is activated. The value can be edited. For Log Normal or Legacy Log Normal distribution, the Median Radius of the particles in the Formulation in µm is activated. The value can be edited. When Tabulated Input Data is selected, this value is fixed based on the Observed Particle Size Distribution data used. |
Standard Deviation | Activated when Distribution Input Data is selected. The Standard Deviation of the radius in µm of the particles in the Formulation. The value can be edited. When Tabulated Input Data is selected, this value is fixed based on the Observed Particle Size Distribution data used. |
Mean Radius | Enabled only when the distribution is Log Normal. The mean radius calculated by the program for use in the Log Normal distribution. |
Number of Bins | The number of sub-compartments into which the particles will be divided according to their radii. All particles within one bin will have the same radius. The mass fraction of the total dose corresponding to each bin will be automatically calculated based on the selected distribution type. |
Radial Cutoff | A toggle. If switched on, the Minimum and Maximum Radius inputs can be edited, and the Automatic Range input is disabled.
If the Radial Cutoff toggle is switched on, these values can be edited.
|
Shape Factor | Specifies the ratio of particle length to its diameter for non-spherical particles. For spherical particles the shape factor = 1. The value can be edited. |
Particle Density | The density, in g/mL, of the particles in the formulation. |
Diffusion Layer | The boundary layer around a dissolving particle or dosage form where the concentration of the solute decreases from that at the surface of the dissolving particle to that in the well-mixed medium and solute transport is primarily diffusion controlled.
The effective particle radius is used as the diffusion layer thickness unless it exceeds the maximum value, in which case the diffusion layer thickness is set to maximum.
|
Tabulated refers to data being presented in tabular form, such as in columns or tables.
Controlled Release Sub-panel
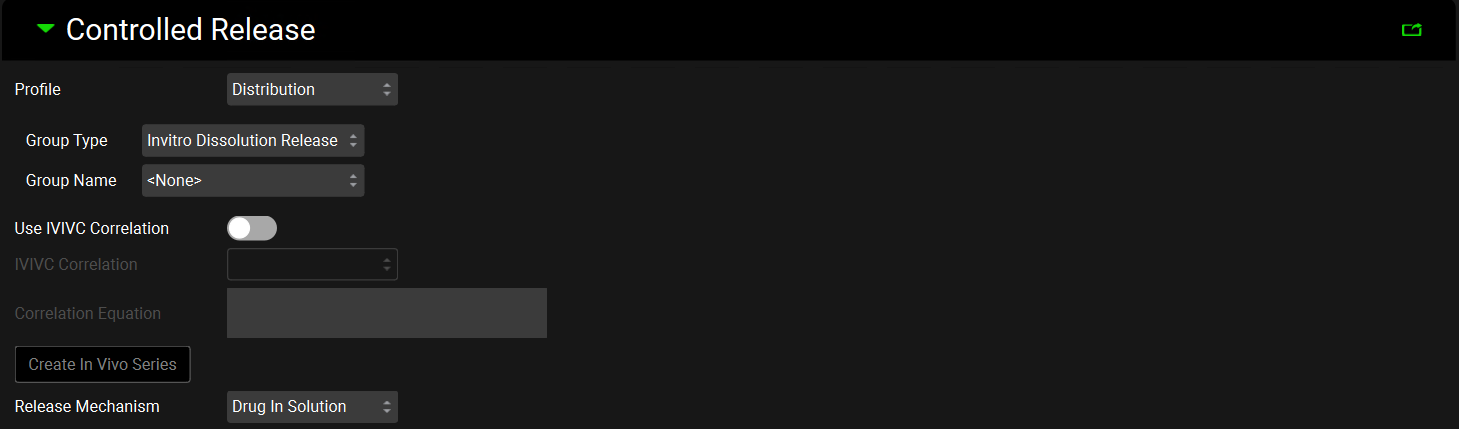
Dosing view, Formulations panel, Controlled Release Sub-panel
Input/Option | Description |
Profile | A drop-down. Selects how the data describing the release will be used. Distribution: uses the Weibull parameters fitted to the Observed Data Group (see Observed Data view, Profiles panel, Distribution options) selected using the Group Type and Group Name below. Tabulated uses the interpolated profile in the Observed Data Group selected using the Group Type, Group Name and Series Name below. |
Group Type | Selects the Observed Data Group Type (see Observed Data View), either In Vivo Controlled Release or In Vitro Dissolution Release that contains the release profile relevant for this specific Formulation. |
Group Name | Selects the Observed Data Group Name that contains the release profile relevant for this specific Formulation. |
Series Name | Selects the Observed Data Series Name that contains the release profile relevant for this specific Formulation. Activated only if Tabulated is selected as the Profile type. |
Use IVIVC Correlation | A toggle. Activated if Tabulated is selected as the Profile type and an IVIVC Correlation has been saved in the project (see IVIVCPlus™ Module). If turned on an IVIVC Correlation is used to describe the release rate. |
IVIVC Correlation | A drop-down. Selects the IVIVC correlation relevant to the specific formulation. |
Correlation Equation | Automatically filled with the correlation equation taken from the Correlation selected above. |
Create In Vivo Series | Click to create an In Vivo Controlled Release Observed Data Type. Navigate to the Observed Data view to see the profile created. The Group and Series names will both have Converted as the suffix. |
Release Mechanism | Determines whether the drug will be released in solution (i.e. as dissolved drug) or as a suspension (i.e. as undissolved drug), which then dissolves according to specified Dissolution Model subpanel in the Simulations view. For example, if the Johnson model is selected, the Nernst-Brunner modification of the Noyes-Whitney equation using the initial particle radius will be used. |
Stomach Retention Time | Time for which the formulation is retained in stomach (applicable for Gastric Release Dose Forms only). |
Solubilizer Sub-panel
The Solubilizer sub-panel is used to input formulation-specific parameters for solubilizers (e.g. cyclodextrin) used as excipients in oral dosage formulations. Solubilizers must first be added in the Compounds view (see Compounds View) and can then be edited for individual formulations here.
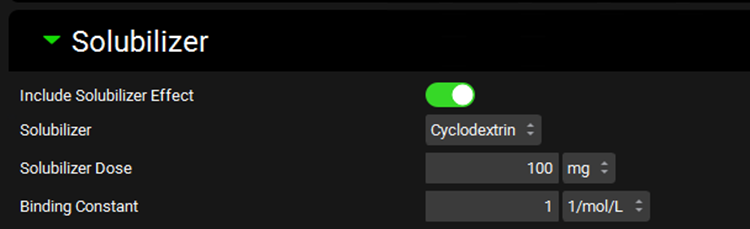
Dosing view, Formulations panel, Solubilizer sub-panel
Input/Option | Description |
Include Solubilizer Effect | A toggle. Turned off by default. Indicates whether solubilizer effects will be included in simulations utilizing dosing schedules which include this formulation. |
Solubilizer | A drop-down. Selects which solubilizer to use with this formulation. Only one solubilizer can be used per formulation. The drop-down list is populated with the Solubilizers added on the Compound view, in the Solubility panel under the Additional Solvents and Solubilizers sub-panel (see Additional Solvents and Solubilizers sub panel). |
Solubilizer Dose | The amount, in mg, of solubilizer included in the formulation. The value can be edited. |
Binding Constant | The binding constant, in (mol/L)-1, of the solubilizer to drug molecules. This controls the rate of disassociation between the compound and the solubilizer during the simulation. The value can be edited. |
Suspension Sub-panel
The Suspension sub-panel is used to enter input formulation-specific parameters for additional suspension vehicles (e.g. ethanol) used as excipients in oral suspension formulations. Solvents must first be added in the Compounds view (see Additional Solvents and Solubilizers sub panel) and can then be edited for individual formulations here.

To simulate an aqueous suspension with a portion of the dose in solution then Simulate Suspension Vehicle needs to be toggled on with a solubility that represents the solubility of the compound in water.
Dosing view, Formulations panel, Suspension Sub-panel
Input/Option | Description |
Simulate Suspension Vehicle | A toggle. Turned on by default for oral suspension formulations. Indicates whether suspension and additional solvent effects will be included in simulations utilizing dosing schedules which include this suspension formulation. If the toggle is off, the compound will be considered as 100% undissolved in the dose vehicle. |
Use Constant Suspension Volume | A toggle. Turned off by default. When it is turned off, the suspension volume will remain constant irrespective of dose, when it is turned on, the volume will scale with the dose strength associated with this formulation in the dosing schedule. |
Constant Vehicle Volume / Vehicle Volume Per Dose | If Use Constant Suspension Volume is toggled on, this will indicate the fixed volume, in mL, of solvent in the formulation. The value can be edited. If the suspension volume represents the entire dose volume, then the dose volume in the Dosing Schedule should be set to 0. If Use Constant Suspension Volume is toggled off, this will indicate the volume of vehicle, in mL/mg, included in the suspension formulation. This is multiplied by the dose strength, in mg, associated with this formulation in the dosing schedule to yield the total volume of solvent. The value can be edited. |
Solvent | A drop-down. Selects which Solvent to use with this formulation. Only one additional solvent can be used per formulation. The drop-down is populated with the Solvents added on the Compound view, in the Solubility panel under the Additional Solvents and Solubilizers sub-panel (see Additional Solvents and Solubilizers sub panel). |
Dosing Schedules Panel
The Dosing Schedules panel is where the formulation(s) are combined with details of the dose amount, volume, and frequency to create a dosing schedule. Dosing schedules are the assets used in simulations to specify the details of drug administration.
Dosing view, Dosing Schedules panel
Input/Option | Description |
Dosing Schedules Name | A drop-down. Selects the dosing schedule.
|
Dose Type | Determines the units of the dose; some of the headings in the table below will adjust to reflect the Dose Type selected.
For PerBodyMass and PerSurfaceArea dose types, the actual dose amount will be calculated when the simulation is run, based on the physiology in that simulation. |
Formulation | A drop-down. Selects which Formulation will be used in this administration in this dose schedule. The drop-down is populated with the Formulations added in the Dosing view, in the Formulations panel. A given dose schedule may have multiple administrations of either the same or different formulations (see below). |
Amount (mg) Amount Per Body Mass (mg/kg) Amount Per Surface Area (mg/m2) | Enter the dose in the units defined by the dose type. The value can be edited. |
Number of Dose Units | Enter the number of units of dose to be administered. The value can be edited. |
Total Dose (mg) Total Dose Per Body Mass (mg/kg) Total Dose Per Surface Area (mg/m2) | The total dose to be administered in the units defined by the dose type. Calculated value defined by multiplying the Amount by the Number of Dose Units. |
Dose Volume (mL) Volume Per Body Mass (mL/kg) Volume Per Surface Area (mL/m2) | The dose volume to be administered (including the amount of water ingested with a tablet or capsule dose) in the units defined by the dose type. The value can be edited. This parameter is applicable only for oral, intramuscular and subcutaneous dosage forms. |
Start | The time of dose administration in hours. The value and units (hours, seconds) can be edited. |
Infusion Duration | The length of an infusion dose in hours. The cell activates only when the formulation is an IntraVenous Infusion dose form. The value and units (seconds, minutes, hours) can be edited. |
Administration Interval | The interval in hours between the dose administrations defined in that row of the table. The value and units (seconds, hours) can be edited. |
Administrations | The number of times the dose defined by that row of the table will be administered. Each administration will be separated by the amount of time specified in the Administration Interval column. The value can be edited. |
Add Dose Administration | A button that adds an additional row to the table above, enabling multiple Formulations and/or dose amounts to be administered in a single Dosing Schedule. |
Delete | A button that deletes the highlighted row in the table above. Hold [CTRL]-key and click to select a row for deletion. |
Repetition of Dosing Schedule | The number of times the entire Dosing Schedule will be repeated. The value can be edited. |
Repeat Dosing Schedule Every | The interval in hours between the repetition of the Dosing Schedule. The value can be edited. |
