PKPlus™ Module
The PKPlus™ module is used for fitting compartmental pharmacokinetic models and conducting noncompartmental analyses using observed plasma concentration-time profiles. Exposure data entered under the Observed Data view (see Profiles Panel) are used as the inputs for PKPlus™ runs. Multiple PKPlus™ runs may be set up within a project, to conduct multiple fittings using either different data or different settings.
PKPlus™ Module
The PKPlus™ module consists of a drop-down at the top for selecting the compound of interest, followed by two panels: Inputs and Runs. The Inputs panel controls setup of exposure data for use in the PKPlus™ runs, while the Runs panel covers settings for the compartmental fitting and noncompartmental analyses, as well as export options for the fitted results.
Inputs panel
The Inputs panel contains a table where individual inputs for PKPlus™ are defined. All inputs are based on series previously added as exposure data in the Observed Data view (see Profiles Panel). In addition to selecting the individual series which will be used, the dose, infusion time, and body weight that correspond to each study are defined here. If these parameters have already been defined in Observed Data, then they will be automatically populated here.
PKPlus™ Module , Inputs panel
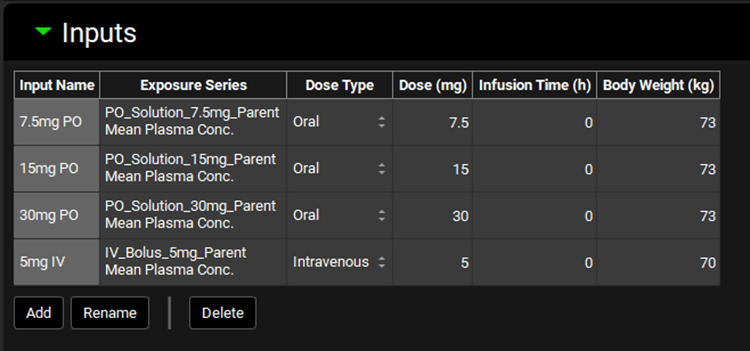
Input/Option | Description |
Input Name | The user-entered name that corresponds to the input represented by the row in the table. |
Exposure Series | The group and series that corresponds to the given input. Selections are made based on groups and series entered under Observed Data. Both the group and the series may be selected by clicking on the cell. |
Dose Type | Select the route of the dose (Oral or Intravenous) in the study that corresponds to the indicated observed data series. |
Dose | The amount of compound, in mg, administered in the study that corresponds to the indicated observed data series. The value can be edited. If this information has already been entered under Observed Data for the selected series, it will be automatically populated here. |
Infusion Time | The infusion time, in hours, over which Intravenous infusion doses were administered in the study that corresponds to the indicated observed data series. The value can be edited. Relevant only for intravenous infusion doses, enter 0 for Intravenous bolus and Oral doses. If this information has already been entered under Observed Data for the selected series, it will be automatically populated here. |
Body Weight | The mass, in kg, of the subject(s) in the study that corresponds to the indicated observed data series. The value can be edited. If multiple subjects are represented in a single series as the mean plasma concentration, this should be the mean body mass. If this information has already been entered under Observed Data for the selected series, it will be automatically populated here. |
Add | Adds a row to the table. Upon clicking Add, users will be prompted to name the input. |
Rename | Renames the currently selected input. Inputs are selected by clicking on the cell. |
Delete | Deletes the currently selected input. Inputs are selected by clicking on the cell. |
PKPlus™ Module, Inputs panel, series selector expanded
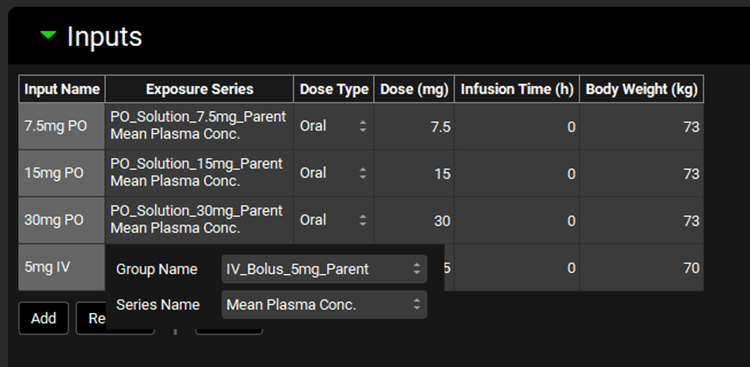
Input/Option | Description |
Group Name | Selects the group that contains relevant observed series. Options are populated based on Exposure Data Groups added in the Observed Data view. |
Series Name | Selects the specific series for the input. Options are populated based on Series added in the Observed Data view to the above selected Group. |
Runs panel
The Runs panel covers the setup of PKPlus™ Runs, which conduct the fitting of compartmental pharmacokinetic models and the noncompartmental analyses. The specific analyses conducted depend on the run settings, which are specified in the Model Parameters sub-panel.
Multiple PKPlus™ Runs may be created and saved, using any combination of inputs defined in the Inputs panel. This permits comparison of different fitting approaches, or quick re-running of PKPlus™ between GPX™ sessions.
After completing a PKPlus™ Run, the results of the fitting are viewed in the Results sub-panel, where they may also be exported to the Compartmental Pharmacokinetics table and to relevant inputs in the Simulations view.
PKPlus™ Module, Runs panel
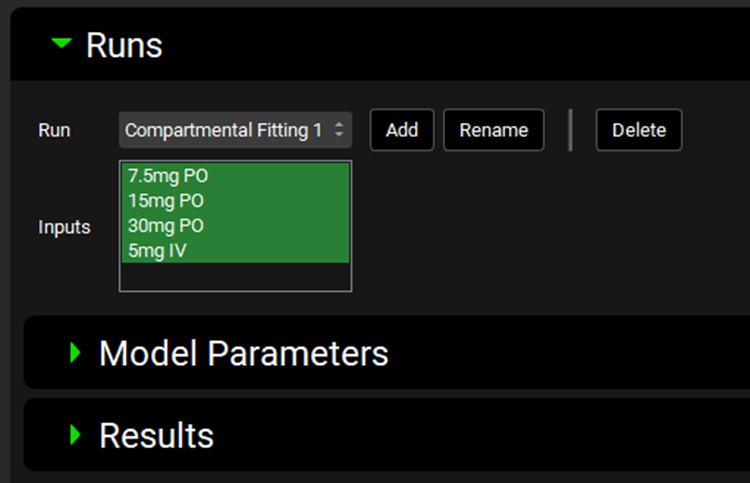
Input/Option | Description |
Run | A drop-down. Selects the current PKPlus™ Run, to which all below settings will apply. This drop-down will be blank until the first PKPlus™ Run is added (see below) |
Add | Add a new PKPlus™ Run, raising an input box where the run can be named. |
Rename | Renames the currently selected PKPlus™ Run |
Delete | Deletes the currently selected PKPlus™ Run |
Inputs | Selects the Inputs that will be used for the current PKPlus™ Run. The options available are based on the inputs defined in the Input panel. Inputs are selected by clicking on the name, and selected entries will be highlighted in green. Multiple inputs can be selected by clicking each required input in turn, or by clicking-and-dragging. |
Model Parameters sub-panel
The Model Parameters sub-panel contains settings for the fitting to be conducted during the PKPlus™ run.
PKPlus™ Module, Runs panel, Model Parameters sub-panel
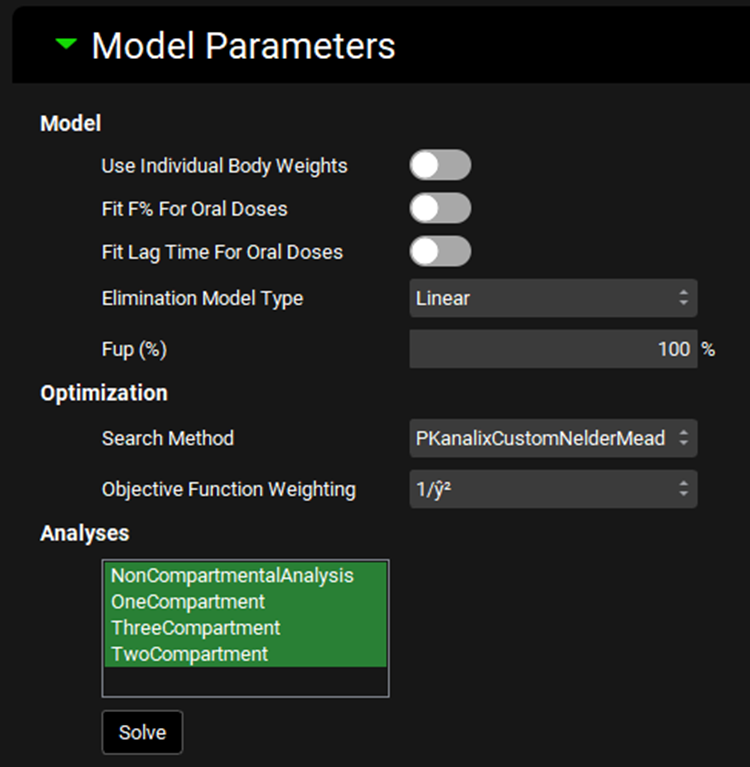
Input/Option | Description |
Model | This section covers general settings for fitting the model, including handling of oral doses and linear versus nonlinear clearance. |
Use Individual Body Weights | A toggle. Controls whether the individual body weights will be used for each input. Relevant for per-body-mass fitted parameters, such as CL/kg. If toggled off, the average of all input body weights will be used during fitting. |
Fit F% For Oral Doses | A toggle. Controls whether bioavailability (F) will be fitted for oral doses. This option requires an intravenous dose input in addition to one or more oral doses. If toggled on, bioavailability will be calculated for oral doses relative to intravenous doses. This permits calculation of first-pass extraction, which can then be exported to simulation settings after fitting (see Results sub-panel below). |
Fit Lag Time For Oral Doses | A toggle. Controls whether a lag time will be fitted for oral doses. If toggled on, lag time will be calculated for oral doses, which can then be exported to simulation settings after fitting (see Results sub-panel below). |
Elimination Model Type | A drop-down. Selects the elimination model to use during fitting: Linear or NonLinear.
|
Fup | The fraction unbound in plasma, as a percentage. This value is used only in the fitting of Km for NonLinear elimination. The value can be edited. The default value is 100%, irrespective of Fup values entered elsewhere in the project. |
Optimization | This section controls settings for the optimization algorithm used in the fitting. |
Search Method | A drop-down. Selects the search algorithm used in the fitting process. The default option is PKanalixCustomNelderMead. The alternative option is NelderMeadSimple. |
Objective Function Weighting | Selects the objective weighting function to be used in the calculation of the objective function. Options are: Uniform (unity), 1/y, 1/y2, 1/ŷ, 1/ŷ2, 1/(y+ ŷ), and 1/(y+ ŷ)2. 1/ŷ2 is the default selection. |
Analyses | Selects the types of analyses to conduct. Options are 1, 2, and 3 compartment models, as well as noncompartmental analysis. Analyses are selected by clicking on the name, and selected entries will be highlighted in green. Multiple analyses can be selected by clicking each required input in turn, or clicking-and-dragging. |
Solve | Runs the PKPlus™ Run to solve the compartmental fitting and noncompartmental analysis, based on the settings and selections defined above. After clicking Solve, the outputs will be populated in the Results sub-panel (see below). |
Results sub-panel
The results sub-panel displays the results of the PKPlus™ run. The tables in this section will be blank until the Solve button is clicked in the Model Parameters sub-panel above. In addition to displaying the results, this panel provides options to export fitted compartmental parameters to the Compartmental Pharmacokinetics table (see Compartmental Panel) and fitted Ka and Time Lag to the Simulations View (see Simplified Absorption sub-panel).
The Non-Compartmental Analysis table displays analysis results for each input.
PKPlus™ Module, Runs panel, Results sub-panel, Non-Compartmental Analysis
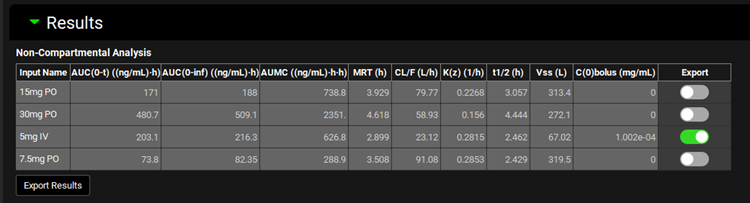
Input/Option | Description |
Input Name | The name of the input included in the PKPlus™ Run, to which the remaining fitted parameters in the table correspond. A separate noncompartmental analysis is conducted for each input. |
AUC(0-t) | The area under the curve, in (ng/mL)•h, of the input, as calculated from time 0 to time t, where time t is the last data point for the series. |
AUC(0-inf) | The area under the curve, in (ng/mL)•h, of the input, extrapolated out to infinity. |
AUMC | The area under the moment curve, in (ng/mL)•h•h, of the input. |
MRT | The mean residence time, in hours, of the input. |
CL/F | The clearance divided by bioavailability, in L/h, of the input. For intravenous doses, bioavailability is 1, and therefore this is equal to clearance. |
K(z) | The elimination rate constant for the terminal phase, in 1/h, for the input. |
t1/2 | The half-life, in h, for the input. |
Vss | The volume of distribution at steady state, in L, for the input. |
C(0) bolus | The concentration at time 0, in mg/mL, for the input. Applies only to intravenous bolus doses. |
Export | A toggle. Controls whether the indicated row will be exported if the Export Results button below the Non-Compartmental Analysis table is clicked. |
Export Results | Exports the selected results (specifically, the row(s) for which Export is toggled On) to the Compartmental Pharmacokinetics table for the compound selected at the top of the PKPlus™ Module view. These will be exported as one compartment models using the Vss as Vc and CL/F as General Clearance. This button will export noncompartmental analyses results only, compartmental fitting results are handled below. |
The Compartmental Analysis table displays results of one, two, and three compartment model fitting (based on the models selected for Analysis in the Model Parameters sub-panel). Different results may be displayed if Elimination Model was linear or nonlinear.
PKPlus™ Module, Runs panel, Results sub-panel, Compartmental Analysis (Linear Elimination)
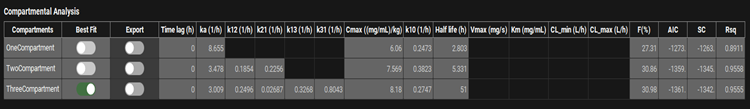
Input/Option | Description |
Compartments | Lists the compartmental models, by number of compartments, selected for fitting. Parameters to the right in each row apply to the indicated compartmental model. |
Best Fit | Indicates which model yields the best fit to the data, based on the AIC (see below). This is provided for information and cannot be changed. |
Export | A toggle. Controls whether the indicated compartmental model will be exported upon clicking of the Export Results button below the plot. |
Time Lag | The lag time, in hours, fitted for oral doses if selected in Model Parameters sub-panel. |
ka | The absorption rate constant (ka), in 1/h, fitted for oral doses. |
k12 | Rate constant for transfer from central compartment to compartment two, in 1/h, fitted for two- and three-compartment models. |
k21 | Rate constant for transfer from compartment two to central compartment, in 1/h, fitted for two- and three-compartment models. |
k13 | Rate constant for transfer from central compartment to compartment three, in 1/h, fitted for three-compartment models. |
k31 | Rate constant for transfer from compartment three to central compartment, in 1/h, fitted for three-compartment models. |
Cmax | The dose normalized Cmax, in mg/mL/kg-dose, of the fitted model. |
k10 | The elimination rate constant (kel), in 1/h, from the central compartment, fitted for one-, two-, and three-compartment models |
Half life | The calculated half-life for the indicated model. |
Vmax, Km, CL_min, CL_max | These columns apply only to nonlinear elimination and will be blank when the Elimination Model is set to Linear. |
F | The calculated bioavailability for oral doses, relative to intravenous administration. Requires both oral and intravenous inputs. |
AIC, SC, and Rsq. | Statistical parameters indicating the goodness-of-fit of each compartmental model. |
PKPlus™ Module, Runs panel, Results sub-panel, Compartmental Analysis (NonLinear Elimination)
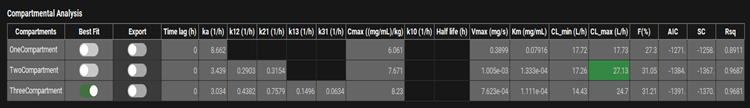
Input/Option | Description |
Compartments | Lists the compartmental models, by number of compartments selected for fitting. Parameters to the right in each row apply to the indicated compartmental model. |
Best Fit | Indicates which model yields the best fit to the data, based on the AIC (see below). This is provided for information and cannot be changed. |
Export | A toggle. Controls whether the indicated compartmental model will be exported upon clicking of the Export Results button below the plot. |
Time Lag | The lag time, in hours, fitted for oral doses if selected in Model Parameters sub-panel. |
ka | The absorption rate constant (ka), in 1/h, fitted for oral doses. |
k12 | Rate constant for transfer from central compartment to compartment two, in 1/h, fitted for two- and three-compartment models. |
k21 | Rate constant for transfer from compartment two to central compartment, in 1/h, fitted for two- and three-compartment models. |
k13 | Rate constant for transfer from central compartment to compartment three, in 1/h, fitted for three-compartment models. |
k31 | Rate constant for transfer from compartment three to central compartment, in 1/h, fitted for three-compartment models. |
Cmax | The dose normalized Cmax, in mg/mL/kg-dose, of the fitted model. |
k10, Half life | These columns apply only to linear elimination and will be blank when the Elimination Model is set to NonLinear. |
Vmax | The Vmax, in mg/s, fitted for the indicated model. |
Km | The Km, in mg/mL, fitted for the indicated model. |
CL_min | The minimum rate of clearance, in L/h, for the indicated model. This value is calculated from Vmax, Km, and the lowest substrate concentration across the prediction. |
CL_max | The maximum rate of clearance, in L/h, for the indicated model. This value is calculated from Vmax, Km, and the highest substrate concentration across the prediction. |
F(%) | The calculated bioavailability for oral doses, relative to intravenous administration. Requires both oral and intravenous inputs. |
AIC, SC, and Rsq. | Statistical parameters indicating the goodness-of-fit of each compartmental model. |
Below the Compartmental Analysis table are two additional tables: Clearance and Volume of Distribution, and Weight Normalized Clearance and Volume of Distribution. These tables display the calculated clearance and volume parameters for each of the compartmental models, based on the fitted parameters in the table above. The first table provides the absolute values, while the second normalizes these values to the subject weight(s) from the PKPlus™ Run inputs.
PKPlus™ Module, Runs panel, Results sub-panel, Clearance and Volume of Distribution
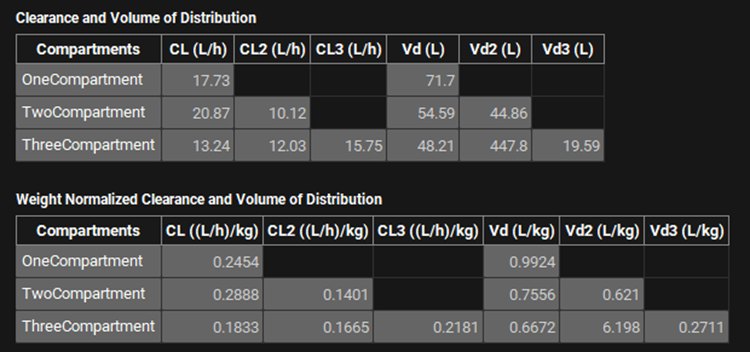
Data can be copied to the clipboard from both the tables and the plot by right clicking anywhere on the table or plot and selecting copy data from the context menu. The image of the plot can be copied or saved as well. This permits easy copying of results to Excel, Word, PowerPoint, etc.
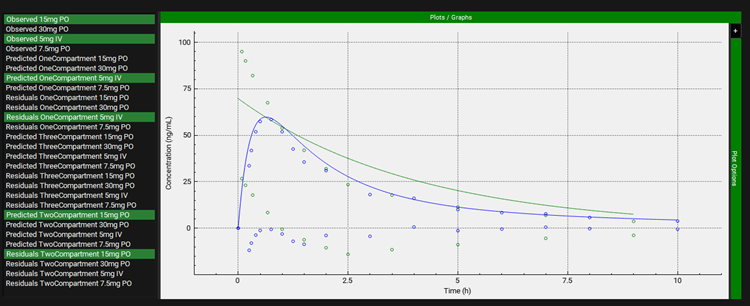
Below these two tables is the PKPlus™ plot, which graphically displays the inputs and outputs of the PKPlus™ Run. The observed data are plotted as points, colored by input. The fitted compartmental models (one-, two-, and three-compartments, based on the Analyses selection in Model Parameters sub-panel) are plotted for each input as solid lines, matched by color to the corresponding input. Lastly, the residuals for each prediction (predicted minus observed) are shown as points, also with matching colors. The specific inputs and outputs displayed are selected on the left-hand side of the plot, with additional options in the expandable Plot Options on the right-hand side. Both of these features behave the same as other plots in GPX™ (see Plot Settings and Plot Options).
PKPlus™ Module, Runs panel, Results sub-panel, PKPlus™ plot (two inputs and corresponding outputs selected)
The final items at the bottom of the PKPlus™ Module view are options for exporting compartmental model results to the Compound assets, and simplified absorption parameters to Simulation assets.
PKPlus™ Module, Runs panel, Results sub-panel, export options
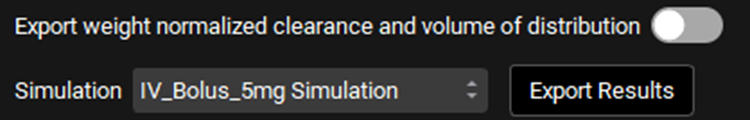
Input/Option | Description |
Export weight normalized clearance and volume of distribution | A toggle. Controls whether fitted compartmental model parameters will be exported from the table with absolute values or with weight normalized values. For example, if toggled Off, clearance would be exported from table with absolute values, whereas if toggled On, clearance would be exported from table with weight normalized values. |
Simulation | A drop-down. selects the simulation to which the Ka, time lag, and F will be exported. Ka and time lag apply to simulations using the Simplified absorption model. F is exported as fixed liver first pass extraction (FPE). Which set of parameters are exported is based on the toggle in the Export column in the Compartmental Analysis table. If multiple rows are selected for export, the values will be averaged. |
Export Results | Exports the results. Ka, time lag, and F are exported to the selected simulation, as described above. Fitted compartmental model parameters are exported to the Compartmental Pharmacokinetics table (see Compartmental Panel) for the compound selected at the top of the PKPlus™ Module. Which compartmental models are exported is based on the toggle in the Export column in the Compartmental Analysis table. The corresponding clearance and volume values are also exported from the Clearance and Volume of Distribution table. If multiple rows are selected for export, multiple models will be exported. |
