AssessmentsPlus™ Module
The AssessmentsPlus™ Module provides modeling advice and insights tailored to a specific compound or simulation. It provides feedback based on modeling experience and scientific heuristics, assessing the expected and actual impact of compound, physiological, and pharmacokinetic properties. This advice can then be used to build or improve pharmacokinetic models in GastroPlus®.
Two types of assessments are currently offered: Compound Assessment and Simulation Assessment. The Compound Assessment is intended to be conducted prior to running a simulation—it will describe what is expected to occur in a simulation using the compound, based on the compound, dosing, physiological, and pharmacokinetic properties provided. It will also highlight any properties or settings that users should pay special attention to when creating their simulation. Additionally, if ADMET Predictor® is licensed, it can be used to compare predicted properties for the compound with those currently entered in the model.
The Simulation Assessment is designed to describe what actually occurred in a simulation, based on both the simulation outputs and the input parameters, and provide advice on how to improve the model and/or drug product. There are multiple modes in which the simulation assessment can be run, which are discussed in more detail below. Broadly, these include improving the fit of the model to observed data, giving advice on how to improve a drug product once a well-fitting model has been developed, or providing a descriptive analysis only.
Once an Assessment is run, the outputs are displayed in a table below the settings. The feedback messages are organized by type and topic, indicate the priority level, and may be color coded to highlight the most salient points. These outputs may be further exported to the AssessmentsPlus™ AI web-app (AssessmentsPlus™ AI), which provides an AI-powered enhancement of the assessment.
The AssessmentsPlus™ Module has four main sections. All the inputs and options on these four sections are described below.
See:
AssessmentsPlus™ Module
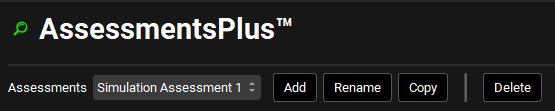
At the top of the AssessmentsPlus™ Module, users can add, select, rename, copy, and delete Assessments. When adding an Assessment, users will decide whether it will be a Compound or Simulation Assessment. The type of Simulation selected will dictate the options available in the panel below.
AssessmentsPlus™ Module
Input/Option | Description |
Assessments | A drop-down. Selects the current assessment. Options are populated based on assessments already added to the project. If no assessments have been added, this will be blank, and the options below will be disabled.
|
Compound Assessment
The Compound Assessment analyzes properties of the selected compound in combination with the indicated physiology, dosing schedule, and pharmacokinetic model, in order to assess how the compound is expected to behave in a simulation. This includes a detailed analysis of the physicochemical properties of the compound, including factors such as ionization, dissolution, and permeability, as well handling BCS, DCS, and property classifications.
Additionally, the assessment examines the selected pharmacokinetic model (including the PEAR physiology for PBPK models) and provides feedback on how distribution and clearance processes are anticipated to behave in the simulation. For PBPK models, this includes an assessment of how physicochemical properties interact with tissue composition and volume to drive distribution, and an evaluation of the contributors to mechanistic clearance.
If selected, the compound assessment will also perform a comparison using ADMET Predictor®, comparing the properties from the compound asset in the project to the predicted properties from ADMET Predictor® for the indicated structure. In addition to a comparison of physicochemical and physiomolecular properties, the enzyme and transporter substrate models in ADMET Predictor® will be used to provide feedback on likely kinetic enterocyte processes and systemic clearance mechanisms. This feature requires a license for the ADMET Predictor® module.
Of note, the Compound Assessment inputs do not include any reference to a Simulation. As such, GastroPlus® parameters that are Simulation Settings, such as dissolution model, solubilization ratio model, enterohepatic recirculation, and fixed first pass extraction, will not be directly considered within the Assessment. However, advice on how to set up Simulation Settings may still be provided, based on the properties of the specific compound.
AssessmentsPlus™ Module, Compound Assessment
Input/Option | Description |
|---|---|
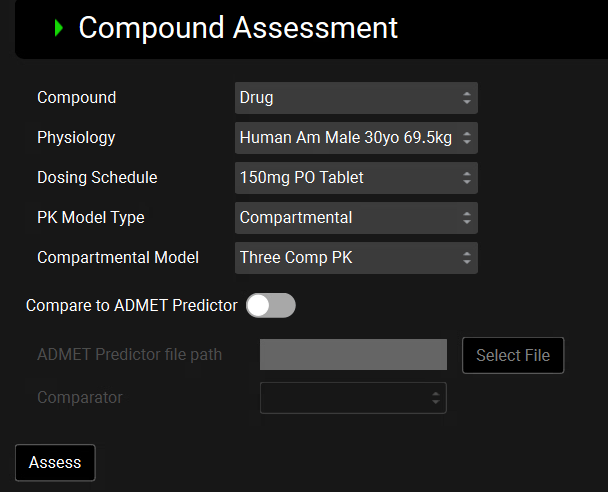
Compound | A drop-down. Selects the compound that will be assessed, in combination with the additional selections below. The compound selected here will dictate what options are available below for Physiology, PK Model Type, and Compartmental Model (if applicable). |
Physiology | A drop-down. Specifies the physiology, and by extension, the physiomolecular properties, which will be used in the assessment. This drop-down is populated with physiologies that have been previously paired with the selected compound. |
Dosing Schedule | A drop-down. Defines the dosing schedule that will be used in this assessment. The list of available dosing schedules is filtered to ensure that the number of forms (molecular polymorphs) in the formulation(s) matches the number of forms setup for the selected compound. |
PK Model Type | A drop-down. Selects the type of pharmacokinetic model to be used in the assessment: Compartmental or Physiologically Based (PBPK). Compartmental will only be an option if the selected compound has one or more compartmental models entered in the Pharmacokinetics view. Physiologically Based will only be an option if the selected physiology has a PEAR physiology created, and the selected compound has a PBPK model setup for this physiology. |
Compartmental Model | A drop-down. Selects which compartmental model will be used in the assessment. This is used only when the PK Model Type is set to Compartmental. |
Compare to ADMET Predictor® | A toggle. Selects whether ADMET Predictor® will be used to provide additional feedback. When toggled ON, the ADMET Predictor® file path and Comparator inputs below will be enabled, and are required to conduct the comparison. |
ADMET Predictor® file path | Selects which structure file will be used for the ADMET Predictor® comparison.
Valid file types include the standard 2D structure files used by ADMET Predictor® (.smi, .mol, .sdf, etc.). The current user preferences will dictate what ADMET Predictor® version will be used. If the structure file contains more than one structure, then the specific structure to use will need to be selected below. |
Comparator | A drop-down. Selects which structure from the above selected structure file will be used for the ADMET Predictor® comparison. If there is only one structure in the selected file, then this will be populated with the identifier for the structure by default, and no further selection need be made. |
Assess | A button. Runs the compound assessment the selected inputs. |
Simulation Assessment
The Simulation Assessment analyzes simulation results, in conjunction with simulation inputs and settings, to describe what occurred in the simulation, explains what pharmacokinetic processes contributed to these results, and provides feedback on how to improve the model or drug product. Simulations need to be set up prior to creating the Simulation Assessment. They do not, however, need to be run independently prior to running the Simulation Assessment—the AssessmentsPlus™ module will run the simulation in the background when conducting the assessment, in order to ensure that the current settings match the outputs, and no stale information is accidentally used.
To ensure that the advice given is relevant and accurate, there are limitations on what kinds of simulations can be assessed. Currently, only single simulations with a single IV or PO dose administration can be used in the AssessmentsPlus™ Module. The Simulation Assessment works under the assumption that systemic exposure is desired.
The Simulation Assessment can be run in various modes, each with a subset of scenarios. The mode and scenario do not change how the simulation is run or how the calculations are performed, but they do control what feedback is generated, tailoring it to the specific scenario at hand. Some modes and scenarios are dependent on the administration route and the presence of mapped observed data. A more detailed description of the available modes and scenarios follows below:
Descriptive Analysis
The Descriptive Analysis mode provides a description of simulation results, along with the pharmacokinetic processes that drove them, without any advice or discussion of recommended actions. The pharmacokinetic domains covered include controlled release, solubility and dissolution, permeability and absorption, first pass extraction, systemic distribution (compartmental or PBPK), clearance (renal, biliary, metabolic, and intrinsic), and enzyme and transporter kinetics, although not all of these topics will be relevant for all simulations. The only scenario applicable for this mode is Descriptive, which can be run on any PO or IV simulation.
Improve Fit to Observed Data
The Improve Fit to Observed Data mode includes everything in the Descriptive Analysis, as well as a comparison of simulated and observed profiles, an assessment of the prediction quality, and advice for improving the fit, if needed. This mode is available for both IV and PO simulations, as long as at least one observed Cp-time profile is mapped in the simulation settings (See Additional options for some Data Types and Observed Data sub-panel). If multiple Cp-time profiles (series) are contained within the mapped group, they will be averaged into a composite profile for the purpose of the assessment. Where calculations on summary pharmacokinetic parameters (e.g. AUC, Cmax, etc.) are required, a noncompartmental analysis (“NCA”) is performed on this profile, as well as on the simulated profile using the subset of points that match observation times.
There are three scenarios available for this mode: Fit Distribution Model to IV Data, and Fit Clearance Model to IV Data, Fit Model to PO Data.
Fit Distribution Model to IV Data: This scenario provides feedback on how to improve the fit of the distribution phase following intravenous drug administration. It assumes that a reasonable clearance value has been set for the model, such as inputting the observed NCA clearance as linear clearance in the pharmacokinetic model used in the simulation. It then proceeds to assess the goodness-of-fit of the distribution phase and identifies processes that may be driving any mismatch, including advice on how to improve the fit.
Fit Clearance Model to IV Data: This scenario provides feedback on how to improve the fit of the clearance phase following intravenous drug administration. It is recommended to run this scenario after running the distribution assessment, or after manually obtaining a good fit for distribution. This assessment analyzes four different routes of clearance—renal, biliary, metabolic, and intrinsic—and provides advice on each, as well as on the overall rate and extent of clearance in the simulation. If available, this assessment will compare the simulated mass cleared by each of these routes to the corresponding observed data profiles, and use this information to refine the feedback, addressing the interplay of multiple clearance mechanisms.
Unique to this scenario, the Compare to ADMET Predictor® option is available. In this case, ADMET Predictor® will be used to predict likely clearance pathways, and this information will be used when observed clearance data (e.g. mass recovered in urine) is not available for a given route of clearance. This feature requires a license for the ADMET Predictor® module.Fit Model to PO Data: This scenario provides feedback on how to improve the fit of an oral simulation. A major focus is on factors affecting absorption and bioavailability, including dissolution, permeability and absorption, and first pass extraction. However, it also addresses distribution and clearance, as applicable.
Drug Product Improvement
The Drug Product Improvement mode includes everything in the Descriptive Analysis, as well as assessment of the impact of absorption, first pass extraction, and bioavailability on clinical variability and regulatory requirements. There are five Scenarios available for this mode, labelled as Development Stages in this context: Lead Selection, Preclinical, Clinical, Generic BE, and Generic 505b2. The advice on what to improve in the drug product is tailored to the stage of development.
This mode assumes that the selected simulation adequately represents the real-world behavior of the compound, and as such does not require any observed data for these predictions.
AssessmentsPlus™ Module, Simulation Assessment
Input/Option | Description |
|---|---|
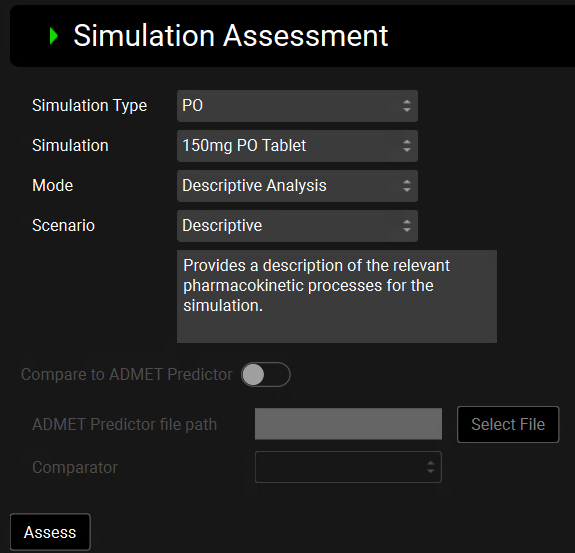
Simulation Type | A drop-down. Selects the route of drug administration in the simulation: IV or PO. This is used to filter the options below for selecting the actual simulation and which modes are available. |
Simulation | A drop-down. Selects the simulation to be used in the assessment. The list is populated from existing simulations in the project, filtered for validity. Valid simulations:
Selecting a simulation without mapped observed data will preclude selection of the Improve Fit to Observed Data mode below. |
Mode | A drop-down. Selects the assessment mode: Descriptive Analysis, Improve Fit to Observed Data, or Drug Product Improvement.
|
Scenario/Development Stage | A drop-down. Allows selection of a predefined scenario or development stage:
|
Scenario/Development Stage Description | A read-only field. Displays explanatory text corresponding to the selected scenario, providing context for how the assessment will interpret pharmacokinetic processes. |
Compare to ADMET Predictor® | A toggle. Selects whether ADMET Predictor® will be used to provide additional feedback. When toggled ON, the ADMET Predictor® file path and Comparator inputs below will be enabled, and are required to conduct the comparison. Enabled only when the scenario is set to “Fit Clearance Model to IV Data”. |
ADMET Predictor® file path | Selects which structure file will be used for the ADMET Predictor® comparison.
Valid file types include the standard 2D structure files used by ADMET Predictor® (.smi, .mol, .sdf, etc.). The current user preferences will dictate what ADMET Predictor® version will be used. If the structure file contains more than one structure, then the specific structure to use will need to be selected below. |
Comparator | A drop-down. Selects which structure from the above selected structure file will be used for the ADMET Predictor® comparison. If there is only one structure in the selected file, then this will be populated with the identifier for the structure by default, and no further selection need be made. |
Assess | A button. Runs the Simulation Assessment with the selected inputs. |
Assessment Outputs
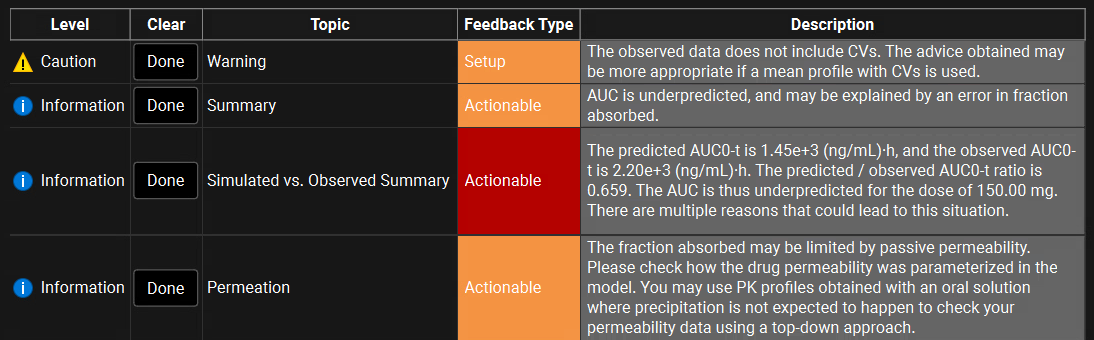
Once the Compound or Simulation Assessment has been run, the results are displayed in a table below the input settings. The output is comprised of a series of messages, contained in this table, each of which has a Level, Topic, Feedback Type, and Description. The Level, Topic, and Feedback Type provide context and organization, while the Description contains the feedback itself. Color coding and icons are used to highlight especially important messages, and the Done button can be used to clear messages once they have been reviewed.
For Compound Assessments, the messages are sorted by topic, then feedback type. For Simulation Assessments, the messages are sorted by feedback type, then topic.
Below the table, there are two buttons to enable use of the web-based AssessmentsPlus™AI application. See AssessmentsPlus™ AI and AssessmentsPlus™ Tutorial: AssessmentsPlus™AI for more information.
AssessmentsPlus™ Module, Assessment Outputs (results shown for an example Simulation Assessment)
Output | Description |
|---|---|
Level | A column displaying the severity or nature of the message. |
Clear | A column with a button ("Done") to dismiss and clear the message. May be used for tracking which messages have been reviewed or resolved. |
Topic | A label indicating to which pharmacokinetic domain the feedback pertains. |
Feedback Type | A label indicating the type of feedback:
High priority messages are color coded to indicate importance and severity:
|
Description | The detailed feedback provided by the assessment. |
AssessmentsPlus™ Module, Assessment Outputs, AssessmentsPlus™ AI
Input/Option | Description |
|---|---|
Copy for AssessmentsPlus™ AI | A button. Copies the assessment outputs as a JSON-formatted string, which serves as the input to AssessmentsPlus™ AI. This string can be copied from GastroPlus® using this button and then pasted into AssessmentsPlus™ AI. Note that this data can also be pasted as plain text into a .json file and saved for later use. |
Launch AssessmentsPlus™ AI | Opens the GastroPlusGPT™ webpage in a new browser window. From here, users can log on and access the AssessmentsPlus™ AI app. |
